Application of a Zwitterionic Hydrophobic Associating Polymer with High Salt and Heat Tolerance in Brine-Based Fracturing Fluid
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of the Hydrophobic Associating Polymer ZID16PM
2.2.1. Synthesis of the Twin-Tailed Hydrophobic Monomer DiC16AM
2.2.2. Synthesis of the ZID16PM
2.3. Experimental Tests
3. Results and Discussion
3.1. Structural Characterization
3.2. Critical Association Concentration
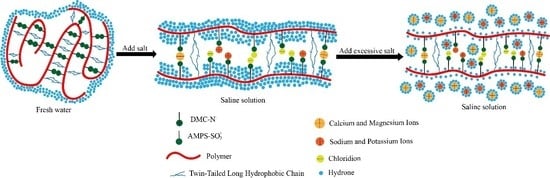
3.3. The Effect of Salt
3.3.1. The Effect of Salt on Viscosity
3.3.2. DLS Measurements
3.4. Evaluation of the Fracturing Fluid Systems
3.4.1. Rheological Measurement
3.4.2. Proppant Suspension Test
3.4.3. Core Matrix Permeability Damage Rate Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Samuel, M.M.; Obianwu, C.W.; Chao, G.W.R.; Samuel, E.; Alim, H.; Hashim, F.; Rohaya, D. An engineered fiber for the fracturing of unconsolidated sand in highly deviated wells in the tali field of brunei. In Proceedings of the European Formation Damage Conference, Scheveningen, The Netherlands, 30 May–1 June 2007. [Google Scholar]
- Waters, G.A.; Dean, B.K.; Downie, R.C.; Kerrihard, K.; Austbo, L.; Mcpherson, B. Simultaneous hydraulic fracturing of adjacent horizontal wells in the woodford shale. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 19–21 January 2009. [Google Scholar]
- Zhao, H.; I-Din, H.A.N.; I-Bagoury, M.A. A new fracturing fluid for hp/ht applications. In Proceedings of the SPE European Formation Damage Conference and Exhibition, Sheveningen, The Netherlands, 25–27 May 2005. [Google Scholar]
- Ellis, R.C. An overview of frac packs: A technical revolution (evolution) process. J. Petrol.Technol. 1998, 50, 66–68. [Google Scholar] [CrossRef]
- Holtsclaw, J.; Funkhouser, G.P. A crosslinkable synthetic-polymer system for high-temperature hydraulic-fracturing applications. SPE Drill. Complet. 2010, 25, 555–563. [Google Scholar] [CrossRef]
- Pu, W.F.; Liu, R.; Wang, K.Y.; Li, K.X.; Yan, Z.P.; Li, B.; Zhao, L. Water-soluble core–shell hyperbranched polymers for enhanced oil recovery. Ind. Eng. Chem. Res. 2015, 54, 798–807. [Google Scholar] [CrossRef]
- Ma, Y. Study on Self-Assembly of Polymer with Hydrophobic Association Containing β Hydroxy Betaine Type Zwitterionic Hydrophobic Associating Polymer and Surfactant. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2016. [Google Scholar]
- Hutchinson, B.H.; Mccormick, C.L. Water-soluble copolymers: 15. studies of random copolymers of acrylamide with n-substituted acrylamides by 13c n.m.r. Polymer 1986, 27, 623–626. [Google Scholar] [CrossRef]
- Klucker, R.; Munch, J.P.; Schosseler, F. Combined static and dynamic light scattering study of associating random block copolymers in solution. Macromolecules 1997, 30, 3839–3848. [Google Scholar] [CrossRef]
- Zhang, L.M. Zwitterionic polymers with Antipolyelectrolyte Behavior in Solution. Polym. Bull. 1998, 82–87. [Google Scholar] [CrossRef]
- Yang, M. On the Effective Utilization of Rainwater Resources, New Silk Road (Second Half); Shaanxi Society for Social Development: Xi’an, China, 2019. [Google Scholar]
- Wu, Y. Current situation and protection of water resources. Charming China 2019, 13, 397–398. [Google Scholar]
- Sina. In a Country Where Water is Scarce and 67 Percent of the Land is Desert, Agriculture is at the Top of the World. Available online: http://k.sina.com.cn/article_6537522375_185aaacc700100c09p.html (accessed on 15 October 2018).
- Sohu. Shilaoren Beach is Black Oil Stick Shoes Wipe off. Available online: http://www.qing5.com/2014/0513/2255.shtml (accessed on 13 May 2014).
- Wang, M.; Wang, K.; Liu, X.; Zhang, J. Experimental study and applications of preparing fracture fluid using re-injected oilfield wastewater. Petrol. Drill. Tech. 2006, 67–70. [Google Scholar]
- Smith, G.L.; Mccormick, C.L. Water-soluble polymers. 80. rheological and photophysical studies of pH-responsive terpolymers containing hydrophobic twin-tailed acrylamide monomers. Macromolecules 2001, 34, 5579–5586. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, J.; Zhao, J.; Yang, X.; Xu, T.; Lin, C.; Mao, J.; Tan, H.; Zhang, Z.; Yang, B.; et al. Preparation of a Hydrophobic-Associating Polymer with Ultra-High Salt Resistance Using Synergistic Effect. Polymers 2019, 11, 626. [Google Scholar] [CrossRef] [Green Version]
- NDAR Commission. Recommended Practices on Measuring the Properties of Waterbased Fracturing Fluid; Chinese Oil and Gas Industry Standards: Beijing, China, 2008. [Google Scholar]
- Lu, D.; Chen, Y.; Xiong, Q. Performance research and recycling of VES fracturing fluid. Petrochem. Technol. 2018, 47, 611–615. [Google Scholar]
- Xin, L.; Yue, X.; Jing, Y.; Zheng, Y. Research on two critical concentrations behavior of a hydrophobic associating copolymer. Chemistry 2005, 68, 793–796. [Google Scholar]
- Zhong, C.; Huang, R.; Xu, J. Characterization, solution behavior, and microstructure of a hydrophobically associating nonionic copolymer. J. Solut. Chem. 2008, 37, 1227–1243. [Google Scholar] [CrossRef]
- Qi, G.; Li, H.; Zhu, R.; Zhang, Z.; Zhou, L.; Kuang, J. Synthesis, characterization, and solution behavior of a long-chain hydrophobic association anionic acrylamide/2-acrylamido-2-methylpropanesulfonic acid/n-octyl acrylate terpolymers. Arab. J. Sci. Eng. 2017, 42, 2425–2432. [Google Scholar] [CrossRef]
- Kaewsaiha, P.; Matsumoto, K.; Matsuoka, H. Non-surface activity and micellization of ionic amphiphilic diblock copolymers in water. hydrophobic chain length dependence and salt effect on surface activity and the critical micelle concentration. Langmuir 2005, 21, 9938–9945. [Google Scholar] [CrossRef]
- Kok, C.M.; Rudin, A. Relationship between the hydrodynamic radius and the radius of gyration of a polymer in solution. Macromol. Rapid Commun. 1981, 2, 655–659. [Google Scholar] [CrossRef]
- Peng, S.; Wu, C. Light scattering study of the formation and structure of partially hydrolyzed poly(acrylamide)/calcium(II) complexes. Macromolecules 1999, 32, 585–589. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Brown, W.; Konak, C. Dynamic light scattering study of the sol-gel transition. Macromolecules 1991, 24, 6839–6842. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Li, J.; Luo, P. Influences of inorganic salts on molecular coil dimension of amphoteric. Oilfield Chemistry 1998, 15, 105–109. [Google Scholar]
- Mccormick, C.L.; Johnson, C.B. Structurally tailored macromolecules for mobility control in enhanced oil recovery. In Water-Soluble Polymers for Petroleum Recovery; Springer: Boston, MA, USA, 1988; pp. 161–180. [Google Scholar]
- Bao, Y.; Ma, J.; Li, N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly(aa-co-am-co-amps)/mmt superabsorbent hydrogel. Carbohydr. Polym. 2011, 84, 76–82. [Google Scholar] [CrossRef]
- Kamal, H.; Hegazy, E.S.A.; Sharada, H.M.; Elhalim, S.A.A.; Lotfy, S.; Mohamed, R.D. Immobilization of glucose isomerase onto radiation synthesized p(aa-co-amps) hydrogel and its application. J. Radiat. Res. Appl. Sci. 2014, 7, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Tan, H.; Yang, B.; Zhang, W.; Yang, X.; Zhang, Y.; Zhang, H. Novel Hydrophobic Associating Polymer with Good Salt Tolerance. Polymers 2018, 10, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, A.; Chowdhuri, S. Pressure effects on the dynamics and hydrogen bond properties of aqueous electrolyte solutions: The role of ion screening. J. Phys. Chem. B 2002, 106, 6779–6783. [Google Scholar] [CrossRef]
- Crozier, P.S.; Rowley, R.L.; Henderson, D. Molecular-dynamics simulations of ion size effects on the fluid structure of aqueous electrolyte systems between charged model electrodes. J. Chem. Phys. 2001, 114, 7513. [Google Scholar] [CrossRef]
- Tansel, B.; Sager, J.; Rector, T.; Garland, J.; Strayer, R.F.; Levine, L.; Roberts, M.; Hummerick, M.; Bauer, J. Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and cross flow modes. Sep. Purif. Technol. 2006, 51, 40–47. [Google Scholar] [CrossRef]
- Hu, J. Study on Formation Water Prepatation of Fracturing Fluid in West Sichuan Gas Field. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2015. [Google Scholar]
- Zhang, W.; Mao, J.; Yang, X.; Zhang, H.; Zhao, J.; Tian, J.; Lin, C.; Mao, J. Development of a sulfonic gemini zwitterionic viscoelastic surfactant with high salt tolerance for seawater-based clean fracturing fluid. Chem. Eng. Sci. 2019, 207, 688–701. [Google Scholar] [CrossRef]
- Yan, Z.; Dai, C.; Zhao, M.; Sun, Y.; Zhao, G. Development, formation mechanism and performance evaluation of a reusable viscoelastic surfactant fracturing fluid. J. Ind. Eng. Chem. 2016, 37, 115–122. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, J.; Zhao, J.; Huang, Y. Damage mechanism of fracturing fluid to reservoir and indoor evaluation and analysis. China Pet. Chem. Stand. Qual. 2013, 159. [Google Scholar] [CrossRef]
- Mao, J.; Tian, J.; Zhang, W.; Yang, X.; Zhang, H.; Lin, C.; Zhang, Y.; Zhang, Z.; Zhao, J. Effects of a counter-ion salt (potassium chloride) on gemini cationic surfactants with different spacer lengths. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123619. [Google Scholar] [CrossRef]













| Component | Content (mg/L) | ||
|---|---|---|---|
| Shanxi | Xinjiang | Jiangsu | |
| K+, Na+ | 6542.685 | 4459 | 4817.01 |
| Ca2+ | 7358.33 | 121.9 | 11.06 |
| Mg2+ | 147.625 | 18.3 | 8.95 |
| Fe | 0.411 | \ | \ |
| SO42− | 509 | 125.9 | 120.18 |
| HCO3− | 201.375 | 1804.4 | 3923.34 |
| CO32− | \ | 237.8 | \ |
| Cl− | 23,269.985 | 5719.8 | 5102.14 |
| TDS | 38,030 | 11,585 | 13,982.68 |
| Fracturing Fluid | Fluid-1 | Fluid-2 | Guar Gum | Linear Glue |
|---|---|---|---|---|
| Settling velocity (mm/s) | 1.45 × 10−3 | 1.98 × 10−3 | 4.41 | 3.26 |
| Fracturing Fluid | Permeability Before Damage (10−3 μm2) | Permeability After Damage (10−3 μm2) | Permeability Damage Rate (%) |
|---|---|---|---|
| Fluid-1 | 10.53 | 9.08 | 13.77 |
| Fluid-2 | 10.76 | 9.51 | 11.62 |
| Guar gum | 11.83 | 8.44 | 28.66 |
| Linear glue | 11.56 | 9.27 | 19.81 |
| SY/T 6376-2008 | \ | \ | ≤30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Mao, J.; Zhang, W.; Yang, X.; Lin, C.; Cun, M.; Mao, J.; Zhao, J. Application of a Zwitterionic Hydrophobic Associating Polymer with High Salt and Heat Tolerance in Brine-Based Fracturing Fluid. Polymers 2019, 11, 2005. https://doi.org/10.3390/polym11122005
Tian J, Mao J, Zhang W, Yang X, Lin C, Cun M, Mao J, Zhao J. Application of a Zwitterionic Hydrophobic Associating Polymer with High Salt and Heat Tolerance in Brine-Based Fracturing Fluid. Polymers. 2019; 11(12):2005. https://doi.org/10.3390/polym11122005
Chicago/Turabian StyleTian, Jizhen, Jincheng Mao, Wenlong Zhang, Xiaojiang Yang, Chong Lin, Meng Cun, Jinhua Mao, and Jinzhou Zhao. 2019. "Application of a Zwitterionic Hydrophobic Associating Polymer with High Salt and Heat Tolerance in Brine-Based Fracturing Fluid" Polymers 11, no. 12: 2005. https://doi.org/10.3390/polym11122005