Recent Developments of the Solution-Processable and Highly Conductive Polyaniline Composites for Optical and Electrochemical Applications
Abstract
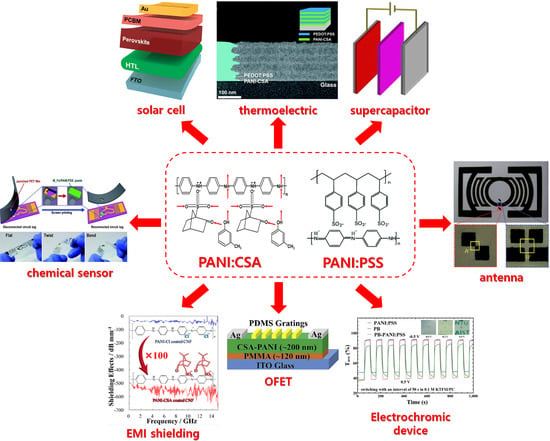
:1. Introduction
2. Solution-Processable PANI Derived from PANI:CSA
2.1. Principle of Secondary Doping of Aniline
2.2. Conductivity Enhancement of PANI:CSA
2.3. Applications of PANI:CSA for Optical and Electrochemical Devices
2.3.1. PANI:CSA for Solar Cell Application
2.3.2. PANI:CSA for PANI:CSA for TE Application
2.3.3. Supercapacitor Application
2.3.4. PANI:CSA for Other Applications: Chemical Sensor, Antenna, EMI Shielding, OFET, and Anti-Corrosion
3. Solution-Processable PANI Derived from Water-Based Systems
3.1. Water-Soluble PANI:PSS for Optical and Electrochemical Applications
3.2. Other Water-Soluble PANI Solutions for Optical and Electrochemical Applications
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Wang, J.; Ou, B.; Zhao, S.; Wang, Z. Some Important Issues of the Commercial Production of 1-D Nano-PANI. Polymers 2019, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kaner, R.B. A General Chemical Route to Polyaniline Nanofibers. J. Am. Chem. Soc. 2004, 126, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Mantione, D.; Del Agua, I.; Sanchez-Sanchez, A.; Mecerreyes, D. Poly(3,4-ethylenedioxythiophene) (PEDOT) Derivatives: Innovative Conductive Polymers for Bioelectronics. Polymers 2017, 9, 354. [Google Scholar] [CrossRef]
- Yan, B.; Wu, Y.; Guo, L. Recent Advances on Polypyrrole Electroactuators. Polymers 2017, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- MacDiarmid, A.G.; Epstein, A.J. Secondary doping in polyaniline. Synth. Met. 1995, 69, 85–92. [Google Scholar] [CrossRef]
- Cho, S.; Kim, M.; Lee, J.S.; Jang, J. Polypropylene/Polyaniline Nanofiber/Reduced Graphene Oxide Nanocomposite with Enhanced Electrical, Dielectric, and Ferroelectric Properties for a High Energy Density Capacitor. ACS Appl. Mater. Interfaces 2015, 7, 22301–22314. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, D.-H.; Lee, K.; Lee, C.-W. High-Performance Polyaniline Prepared via Polymerization in a Self-Stabilized Dispersion. Adv. Funct. Mater. 2005, 15, 1495–1500. [Google Scholar] [CrossRef]
- Kim, M.; Cho, S.; Song, J.; Son, S.; Jang, J. Controllable Synthesis of Highly Conductive Polyaniline Coated Silica Nanoparticles Using Self-Stabilized Dispersion Polymerization. ACS Appl. Mater. Interfaces 2012, 4, 4603–4609. [Google Scholar] [CrossRef]
- Lee, K.; Cho, S.; Heum Park, S.; Heeger, A.J.; Lee, C.-W.; Lee, S.-H. Metallic transport in polyaniline. Nature 2006, 441, 65–68. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Katunin, A. The effect of reaction medium on the conductivity and morphology of polyaniline doped with camphorsulfonic acid. Synth. Met. 2016, 214, 45–49. [Google Scholar] [CrossRef]
- Rannou, P.; Nechtschein, M.; Travers, J.P.; Berner, D.; Woher, A.; Djurado, D. Ageing of PANI: Chemical, structural and transport consequences. Synth. Met. 1999, 101, 734–737. [Google Scholar] [CrossRef]
- Lee, B.H.; Park, S.H.; Back, H.; Lee, K. Novel Film-Casting Method for High-Performance Flexible Polymer Electrodes. Adv. Funct. Mater. 2011, 21, 487–493. [Google Scholar] [CrossRef]
- Jeon, S.S.; Kim, C.; Lee, T.H.; Lee, Y.W.; Do, K.; Ko, J.; Im, S.S. Camphorsulfonic Acid-Doped Polyaniline Transparent Counter Electrode for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2012, 116, 22743–22748. [Google Scholar] [CrossRef]
- Park, S.H.; Shin, K.-H.; Kim, J.-Y.; Yoo, S.J.; Lee, K.J.; Shin, J.; Choi, J.W.; Jang, J.; Sung, Y.-E. The application of camphorsulfonic acid doped polyaniline films prepared on TCO-free glass for counter electrode of bifacial dye-sensitized solar cells. J. Photochem. Photobiol. 2012, 245, 1–8. [Google Scholar] [CrossRef]
- Cho, S.; Hwang, S.H.; Kim, C.; Jang, J. Polyaniline porous counter-electrodes for high performance dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 12164–12171. [Google Scholar] [CrossRef]
- Cho, S.; Shin, K.-H.; Jang, J. Enhanced Electrochemical Performance of Highly Porous Supercapacitor Electrodes Based on Solution Processed Polyaniline Thin Films. ACS Appl. Mater. Interfaces 2013, 5, 9186–9193. [Google Scholar] [CrossRef]
- Lee, K.; Cho, S.; Kim, M.; Kim, J.; Ryu, J.; Shin, K.-Y.; Jang, J. Highly porous nanostructured polyaniline/carbon nanodots as efficient counter electrodes for Pt-free dye-sensitized solar cells. J. Mater. Chem. A 2015, 3, 19018–19026. [Google Scholar] [CrossRef]
- Abdulrazzaq, O.; Bourdo, S.E.; Woo, M.; Saini, V.; Berry, B.C.; Ghosh, A.; Biris, A.S. Comparative Aging Study of Organic Solar Cells Utilizing Polyaniline and PEDOT:PSS as Hole Transport Layers. ACS Appl. Mater. Interfaces 2015, 7, 27667–27675. [Google Scholar] [CrossRef]
- Lee, K.; Yu, H.; Lee, J.W.; Oh, J.; Bae, S.; Kim, S.K.; Jang, J. Efficient and moisture-resistant hole transport layer for inverted perovskite solar cells using solution-processed polyaniline. J. Mater. Chem. C 2018, 6, 6250–6256. [Google Scholar] [CrossRef]
- Lee, H.J.; Anoop, G.; Lee, H.J.; Kim, C.; Park, J.W.; Choi, J.; Kim, H.; Kim, Y.J.; Lee, E.; Lee, S.G.; et al. Enhanced thermoelectric performance of PEDOT:PSS/PANI–CSA polymer multilayer structures. Energy Environ. Sci. 2016, 9, 2806–2811. [Google Scholar] [CrossRef]
- Cho, S.; Kim, M.; Jang, J. Screen-Printable and Flexible RuO2 Nanoparticle-Decorated PEDOT:PSS/Graphene Nanocomposite with Enhanced Electrical and Electrochemical Performances for High-Capacity Supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 10213–10227. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.S.; Jun, J.; Kim, S.G.; Jang, J. Fabrication of water-dispersible and highly conductive PSS-doped PANI/graphene nanocomposites using a high-molecular weight PSS dopant and their application in H2S detection. Nanoscale 2014, 6, 15181–15195. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Cho, K.H.; Ryu, J.; Yun, J.; Yu, H.; Lee, J.; Na, W.; Jang, J. Low-cost and efficient perovskite solar cells using a surfactant-modified polyaniline:poly(styrenesulfonate) hole transport material. Electrochim. Acta 2017, 224, 600–607. [Google Scholar] [CrossRef]
- Jang, J.; Ha, J.; Cho, J. Fabrication of Water-Dispersible Polyaniline-Poly(4-styrenesulfonate) Nanoparticles for Inkjet-Printed Chemical-Sensor Applications. Adv. Mater. 2007, 19, 1772–1775. [Google Scholar] [CrossRef]
- Yoo, J.E.; Lee, K.S.; Garcia, A.; Tarver, J.; Gomez, E.D.; Baldwin, K.; Sun, Y.; Meng, H.; Nguyen, T.Q.; Loo, Y.L. Directly patternable, highly conducting polymers for broad applications in organic electronics. Proc. Natl. Acad. Sci. USA 2010, 107, 5712. [Google Scholar] [CrossRef] [PubMed]
- Isakova, A.; Topham, P.D. Polymer strategies in perovskite solar cells. J. Polym. Sci. Pol. Phys. 2017, 55, 549–568. [Google Scholar] [CrossRef]
- Ecker, B.; Posdorfer, J.; von Hauff, E. Influence of hole extraction efficiency on the performance and stability of organic solar Cells. Sol. Energy Mater. Sol. Cells 2013, 116, 176–181. [Google Scholar] [CrossRef]
- Hu, C.-W.; Kawamoto, T.; Tanaka, H.; Takahashi, A.; Lee, K.-M.; Kao, S.-Y.; Liao, Y.-C.; Ho, K.-C. Water processable Prussian blue–polyaniline:polystyrene sulfonate nanocomposite (PB–PANI:PSS) for multi-color electrochromic applications. J. Mater. Chem. C 2016, 4, 10293–10300. [Google Scholar] [CrossRef]
- Huang, L.M.; Hu, C.W.; Peng, C.Y.; Su, C.H.; Ho, K.C. Integration of polyelectrolyte based electrochromic material in printable photovoltaic electrochromic module. Sol. Energy Mater. Sol. Cells 2016, 145, 69–75. [Google Scholar] [CrossRef]
- Xiong, S.; Lan, J.; Yin, S.; Wang, Y.; Kong, Z.; Gong, M.; Wu, B.; Chu, J.; Wang, X.; Zhang, R.; et al. Enhancing the electrochromic properties of polyaniline via coordinate bond tethering the polyaniline with gold colloids. Sol. Energy Mater. Sol. Cells 2018, 177, 134–141. [Google Scholar] [CrossRef]
- Fenoy, G.E.; Van der Schueren, B.; Scotto, J.; Boulmedais, F.; Ceolín, M.R.; Bégin-Colin, S.; Bégin, D.; Marmisollé, W.A.; Azzaroni, O. Layer-by-layer assembly of iron oxide-decorated few-layer graphene/PANI:PSS composite films for high performance supercapacitors operating in neutral aqueous electrolytes. Electrochim. Acta 2018, 283, 1178–1187. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Hu, N.; Yang, Z.; Wei, H.; Xu, Z.J.; Wang, Y.; Zhang, Y. Densely-packed graphene/conducting polymer nanoparticle papers for high-volumetric-performance flexible all-solid-state supercapacitors. Appl. Surf. Sci. 2016, 379, 206–212. [Google Scholar] [CrossRef]
- Cho, E.-C.; Chang-Jian, C.-W.; Lee, K.-C.; Huang, J.-H.; Ho, B.-C.; Ding, Y.-R.; Hsiao, Y.-S. Spray-dried nanoporous NiO/PANI:PSS composite microspheres for high-performance asymmetric supercapacitors. Compos. B Eng. 2019, 175, 107066. [Google Scholar] [CrossRef]
- Huang, H.; Yao, J.; Chen, H.; Zeng, X.; Chen, C.; She, X.; Li, L. Facile preparation of halloysite/polyaniline nanocomposites via in situ polymerization and layer-by-layer assembly with good supercapacitor performance. J. Mater. Sci. 2016, 51, 4047–4054. [Google Scholar] [CrossRef]
- Ranka, P.; Sethi, V.; Contractor, A.Q. One step electrodeposition of composite of PANI-PSS tubules with TiO2 nanoparticles and application as electronic sensor device. Sens. Actuator B Chem. 2018, 261, 11–21. [Google Scholar] [CrossRef]
- Kim, S.G.; Jun, J.; Lee, J.S.; Jang, J. A highly sensitive wireless nitrogen dioxide gas sensor based on an organic conductive nanocomposite paste. J. Mater. Chem. A 2019, 7, 8451–8459. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, M.; Lee, C.; Cho, S.; Oh, J.; Jang, J. Platinum-decorated reduced graphene oxide/polyaniline:poly(4-styrenesulfonate) hybrid paste for flexible dipole tag-antenna applications. Nanoscale 2015, 7, 3668–3674. [Google Scholar] [CrossRef]
- Han, H.; Lee, J.S.; Cho, S. Comparative Studies on Two-Electrode Symmetric Supercapacitors Based on Polypyrrole:Poly(4-styrenesulfonate) with Different Molecular Weights of Poly(4-styrenesulfonate). Polymers 2019, 11, 232. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.S.; Jee, C.; Bae, J.-H.; Kim, E.; Jung, H.J.; Yang, J.Y.; Huh, P. Semi-crystalline polypyrrole: Polystyrene sulfonate synthesized through the pores of filter paper. Polym. Eng. Sci. 2018, 58, 1033–1036. [Google Scholar] [CrossRef]
- Pattananuwat, P.; Tagaya, M.; Kobayashi, T. Controllable nanoporous fibril-like morphology by layer-by- layer self-assembled films of bioelectronics poly(pyrrole-co-formyl pyrrole)/polystyrene sulfonate for biocompatible electrode. Mater. Res. Bull. 2018, 99, 260–267. [Google Scholar] [CrossRef]
- Bilal, S.; Fahim, M.; Firdous, I.; Ali Shah, A.-u.-H. Insight into capacitive performance of polyaniline/graphene oxide composites with ecofriendly binder. Appl. Surf. Sci. 2018, 435, 91–101. [Google Scholar] [CrossRef]
- Tanzifi, M.; Tavakkoli Yaraki, M.; Karami, M.; Karimi, S.; Dehghani Kiadehi, A.; Karimipour, K.; Wang, S. Modelling of dye adsorption from aqueous solution on polyaniline/carboxymethyl cellulose/TiO2 nanocomposites. J. Colloid Interface Sci. 2018, 519, 154–173. [Google Scholar] [CrossRef] [PubMed]
- De León-Almazán, C.M.; Onofre-Bustamante, E.; Rivera-Armenta, J.L.; Ángeles San Martín, M.E.; Chávez-Cinco, M.Y.; Gallardo-Rivas, N.V.; Páramo-García, U. PAni/SBR composite as anticorrosive coating for carbon steel, part II: Electrochemical characterization. Polym. Bull. 2017, 74, 1145–1155. [Google Scholar] [CrossRef]
- Li, J.-T.; Wu, Z.-Y.; Lu, Y.-Q.; Zhou, Y.; Huang, Q.-S.; Huang, L.; Sun, S.-G. Water Soluble Binder, an Electrochemical Performance Booster for Electrode Materials with High Energy Density. Adv. Energ. Mater. 2017, 7, 1701185. [Google Scholar] [CrossRef]
- Song, B.; Wu, F.; Zhu, Y.; Hou, Z.; Moon, K.-s.; Wong, C.-P. Effect of polymer binders on graphene-based free-standing electrodes for supercapacitors. Electrochim. Acta 2018, 267, 213–221. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, S.; Vongehr, S.; Ali Syed, J.; Wang, X.; Meng, X. High-Performance Flexible Solid-State Carbon Cloth Supercapacitors Based on Highly Processible N-Graphene Doped Polyacrylic Acid/Polyaniline Composites. Sci. Rep. 2016, 6, 12883. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wu, J.; Lan, Z.; Zheng, M.; Yue, G.; Lin, J.; Huang, M. Preparation of PAA-g-PEG/PANI polymer gel electrolyte and its application in quasi solid state dye-sensitized solar cells. Polym. Eng. Sci. 2015, 55, 322–326. [Google Scholar] [CrossRef]
- Homma, T.; Kondo, M.; Kuwahara, T.; Shimomura, M. Polyaniline/poly(acrylic acid) composite film: A promising material for enzyme-aided electrochemical sensors. Eur. Polym. J. 2015, 62, 139–144. [Google Scholar] [CrossRef]
- Duan, Y.; Tang, Q.; Chen, Y.; Zhao, Z.; Lv, Y.; Hou, M.; Yang, P.; He, B.; Yu, L. Solid-state dye-sensitized solar cells from poly(ethylene oxide)/polyaniline electrolytes with catalytic and hole-transporting characteristics. J. Mater. Chem. A 2015, 3, 5368–5374. [Google Scholar] [CrossRef]
- Rijos, L.M.; Melendez, A.; Oyola, R.; Pinto, N.J. Effect of polyethylene oxide on camphor sulfonic acid doped polyaniline thin film field effect transistor with ionic liquid gating. Synth. Met. 2019, 257, 116176. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Tseng, L.-C.; Lee, R.-H. Graphene oxide sheet–polyaniline nanohybrids for enhanced photovoltaic performance of dye-sensitized solar cells. J. Polym. Sci. Pol. Phys. 2014, 52, 321–332. [Google Scholar] [CrossRef]
- Gao, J.; Yang, Y.; Zhang, Z.; Yan, J.; Lin, Z.; Guo, X. Bifacial quasi-solid-state dye-sensitized solar cells with Poly(vinyl pyrrolidone)/polyaniline transparent counter electrode. Nano Energy 2016, 26, 123–130. [Google Scholar] [CrossRef]
- Li, W.; Gao, F.; Wang, X.; Zhang, N.; Ma, M. Strong and Robust Polyaniline-Based Supramolecular Hydrogels for Flexible Supercapacitors. Angew. Chem. 2016, 55, 9196–9201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.M.; Deng, Y. Flexible low-grade energy utilization devices based on high-performance thermoelectric polyaniline/tellurium nanorod hybrid films. J. Mater. Chem. A 2016, 4, 3554–3559. [Google Scholar] [CrossRef]
- Anno, H.; Yamaguchi, K.; Nakabayashi, T.; Kurokawa, H.; Akagi, F.; Hojo, M.; Toshima, N. Thermoelectric properties of conducting polyaniline/BaTiO3 nanoparticle composite films. IOP Conf. Ser. Mater. Sci. Eng. 2011, 18, 142003. [Google Scholar] [CrossRef]
- Khuspe, G.D.; Navale, S.T.; Chougule, M.A.; Patil, V.B. Ammonia gas sensing properties of CSA doped PANi-SnO2 nanohybrid thin films. Synth. Met. 2013, 185–186, 1–8. [Google Scholar] [CrossRef]
- Lee, R.-H.; Chi, C.-H.; Hsu, Y.-C. Platinum nanoparticle/self-doping polyaniline composite-based counter electrodes for dye-sensitized solar cells. J. Nanopart. Res. 2013, 15, 1733. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Li, P.; Yuan, D.; Zhou, X.; Sun, J.; Lu, X.; He, C. Interfacial control and carrier tuning of carbon nanotube/polyaniline composites for high thermoelectric performance. Carbon 2018, 136, 292–298. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, Q.; Wang, L.; Chen, L. Abnormally enhanced thermoelectric transport properties of SWNT/PANI hybrid films by the strengthened PANI molecular ordering. Energy Environ. Sci. 2014, 7, 3801–3807. [Google Scholar] [CrossRef]
- Liu, F.; Luo, S.; Liu, D.; Chen, W.; Huang, Y.; Dong, L.; Wang, L. Facile Processing of Free-Standing Polyaniline/SWCNT Film as an Integrated Electrode for Flexible Supercapacitor Application. ACS Appl. Mater. Interfaces 2017, 9, 33791–33801. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Q.; Bi, H.; Huang, F.; Wang, Q.; Chen, L. PANI/graphene nanocomposite films with high thermoelectric properties by enhanced molecular ordering. J. Mater. Chem. A 2015, 3, 7086–7092. [Google Scholar] [CrossRef]
- Kim, M.; Lee, C.; Jang, J. Fabrication of Highly Flexible, Scalable, and High-Performance Supercapacitors Using Polyaniline/Reduced Graphene Oxide Film with Enhanced Electrical Conductivity and Crystallinity. Adv. Funct. Mater. 2014, 24, 2489–2499. [Google Scholar] [CrossRef]
- Shin, K.-Y.; Kim, M.; Lee, J.S.; Jang, J. Highly Omnidirectional and Frequency Controllable Carbon/Polyaniline-based 2D and 3D Monopole Antenna. Sci. Rep. 2015, 5, 13615. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-C.; Chen, G.-L.; Lee, R.-H. Graphene oxide sheet-polyaniline nanocomposite prepared through in-situ polymerization/deposition method for counter electrode of dye-sensitized solar cell. J. Polym. Res. 2014, 21, 440. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Rashid, S.A.; Abu Bakar, M.H.; Ahmad Anas, S.B.; Mahdi, M.A.; Yaacob, M.H. Fabrication and Characterizations of a Novel Etched-tapered Single Mode Optical Fiber Ammonia Sensors Integrating PANI/GNF Nanocomposite. Sens. Actuator B Chem. 2019, 287, 71–77. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, M.; Oh, J.; Kim, J.; Cho, S.; Jun, J.; Jang, J. Platinum-decorated carbon nanoparticle/polyaniline hybrid paste for flexible wideband dipole tag-antenna application. J. Mater. Chem. A 2015, 3, 7029–7035. [Google Scholar] [CrossRef]
- Oh, J.; Kim, Y.K.; Lee, J.S.; Jang, J. Highly porous structured polyaniline nanocomposites for scalable and flexible high-performance supercapacitors. Nanoscale 2019, 11, 6462–6470. [Google Scholar] [CrossRef]
- Yoon, C.O.; Reghu, M.; Moses, D.; Cao, Y.; Heeger, A.J. Thermoelectric power of doped polyaniline near the metal-insulator transition. Synth. Met. 1995, 69, 273–274. [Google Scholar] [CrossRef]
- Anno, H.; Hokazono, M.; Akagi, F.; Hojo, M.; Toshima, N. Thermoelectric Properties of Polyaniline Films with Different Doping Concentrations of (±)-10-Camphorsulfonic Acid. J. Electron. Mater. 2013, 42, 1346–1351. [Google Scholar] [CrossRef]
- Verma, D.; Dutta, V. Role of novel microstructure of polyaniline-CSA thin film in ammonia sensing at room temperature. Sens. Actuator B Chem. 2008, 134, 373–376. [Google Scholar] [CrossRef]
- Omura, T.; Chan, C.H.; Wakisaka, M.; Nishida, H. Organic Thin Paper of Cellulose Nanofiber/Polyaniline Doped with (±)-10-Camphorsulfonic Acid Nanohybrid and Its Application to Electromagnetic Shielding. ACS Omega 2019, 4, 9446–9452. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Khosla, R.; Das, S.; Shrimali, H.; Sharma, S.K. High-performance CSA-PANI based organic phototransistor by elastomer gratings. Org. Electron. 2018, 57, 14–20. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, Y.; Zhang, Z.; Yu, L. Corrosion protection of a novel SiO2@PANI coating for Q235 carbon steel. Prog. Org. Coat. 2019, 132, 227–234. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Lee, J.S.; Joo, H. Recent Developments of the Solution-Processable and Highly Conductive Polyaniline Composites for Optical and Electrochemical Applications. Polymers 2019, 11, 1965. https://doi.org/10.3390/polym11121965
Cho S, Lee JS, Joo H. Recent Developments of the Solution-Processable and Highly Conductive Polyaniline Composites for Optical and Electrochemical Applications. Polymers. 2019; 11(12):1965. https://doi.org/10.3390/polym11121965
Chicago/Turabian StyleCho, Sunghun, Jun Seop Lee, and Hyeonseo Joo. 2019. "Recent Developments of the Solution-Processable and Highly Conductive Polyaniline Composites for Optical and Electrochemical Applications" Polymers 11, no. 12: 1965. https://doi.org/10.3390/polym11121965