Preparation of Chitosan Stacking Membranes for Adsorption of Copper Ions
Abstract
:1. Introduction
2. Experiment
2.1. Materials and Chemicals
2.2. Properties of Electrospinning and Electrospraying Solutions
2.3. The Fabrication of Monolayer Membrane
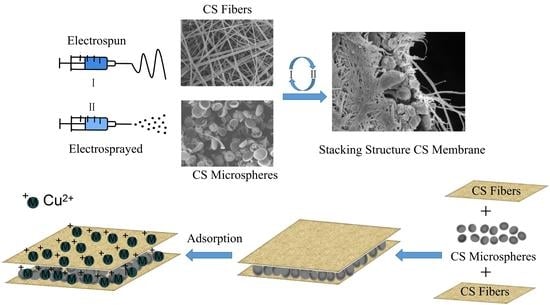
2.4. The CS Stacking Memebranes by Alternated Electrospining/Electrospraying
2.5. Nanofibers Neutralization Treatment
2.6. Characterization of CS Stacking Membranes
2.7. Adsorption Experiments
3. Results and Discussion
3.1. Morphology of Electrospun/Electrosprayed CS Layers
3.2. The Preparation of CS Stacking Membranes
3.3. The Stability in Aqueous
3.4. BET Analysis
3.5. Adsorption Perfomance
3.6. The Equilibrium Isotherm and Rate Constant Studies
3.7. EDS Analysis
3.8. Adsorption Mechanism of Cu(II) by CS Stacking Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fu, F.L.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Asiabi, H.; Yamini, Y.; Shamsayei, M.; Molaei, K.; Shamsipur, M. Functionalized layered double hydroxide with nitrogen and sulfur co-decorated carbondots for highly selective and efficient removal of soft Hg2+ and Ag+ ions. J. Hazard. Mater. 2018, 357, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.N.; Toufeek, M.E.F.; Eltaher, M.A.E.; Elbadry, A.O. Adsorption and leaching behavior of copper, zinc and lead ions by three different river nile sediments at Aswan, Egypt. Pollution 2018, 5, 99–114. [Google Scholar]
- Shi, T.; Jia, S.J.; Chen, Y.; Wen, Y.H.; Du, C.G.; Guo, H.L.; Wang, Z.C. Adsorption of Pb(II), Cr(III), Cu(II), Cd(II) and Ni(II) onto a vanadium mine tailing from aqueous solution. J. Hazard. Mater. 2009, 169, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Kiran, B.; Rani, S.; Rani, A.; Kaur, B.; Mittal, N. Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem. 2008, 111, 811–815. [Google Scholar] [CrossRef]
- Ho, Y.S.; Huang, C.T.; Huang, H.W. Equilibrium sorption isotherm for metal ions on tree fern. Process Biochem. 2002, 37, 1421–1430. [Google Scholar] [CrossRef]
- Theophanides, T.; Anastassopoulou, J. Copper and carcinogenesis. Crit. Rev. Oncol./Hematol. 2002, 42, 57–64. [Google Scholar] [CrossRef]
- Mauchauffée, S.; Meux, E. Use of sodium decanoate for selective precipitation of metals contained in industrial wastewater. Chemosphere 2007, 69, 763–768. [Google Scholar] [CrossRef]
- Samrani, A.G.E.; Lartiges, B.S.; Villiéras, F. Chemical coagulation of combined sewer overflow: Heavy metal removal and treatment optimization. Water Res. 2008, 42, 951–960. [Google Scholar] [CrossRef]
- Rengaraj, S.; Joo, C.K.; Kim, Y.; Yi, J. Kinetics of removal of chromium from water and electronic process wastewater by ion exchange resins: 1200H, 1500H and IRN97H. J. Hazard. Mater. 2003, 102, 257–275. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Li, Y.; Yang, R.; Wang, C. Branched polyethylenimine grafted electrospun polyacrylonitrile fiber membrane: A novel and effective adsorbent for Cr(VI) remediation in wastewater. J. Mater. Chem. A 2017, 5, 1133–1144. [Google Scholar] [CrossRef]
- Almasian, A.; Najafi, F.; Maleknia, L.; Giahi, M. Mesoporous MgO/PPG hybrid nanofibers: Synthesis, optimization, characterization and heavy metal removal property. New J. Chem. 2018, 42, 2013–2029. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Ramin, S. Effective removal of copper from aqueous solutions by modified magnetic chitosan/graphene oxide nanocomposites. Int. J. Biol. Macromol. 2018, 113, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Kaveeshwar, A.R.; Kumar, P.S.; Revellame, E.D.; Gang, D.D.; Zappi, M.; Subramaniam, R. Adsorption properties and mechanism of barium (II) and strontium (II) removal from fracking wastewater using pecan shell based activated carbon. J. Clean. Prod. 2018, 193, 1–13. [Google Scholar] [CrossRef]
- Xin, S.; Zeng, Z.; Zhou, X.; Luo, W.J.; Shi, X.W.; Wang, Q.; Deng, H.; Du, Y.M. Recyclable Saccharomyces cerevisiae loaded nanofibrous mats with sandwich structure constructing via bio-electrospraying for heavy metal removal. J. Hazard. Mater. 2017, 324, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Taimur, S.; Hassan, M.I.; Yasin, T. Removal of copper using novel amidoxime based chelating nanohybrid adsorbent. Eur. Polym. J. 2017, 95, 93–104. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, R.; Chao, S. Polydopamine coating assisted synthesis of MnO2, loaded inorganic/organic composite electrospun fiber adsorbent for efficient removal of Pb2+, from water. Chem. Eng. J. 2018, 344, 277–289. [Google Scholar] [CrossRef]
- Vu, D.; Li, Z.Y.; Zhang, H.G.; Wang, W.; Wang, Z.J.; Xu, X.; Dong, B.; Wang, C. Adsorption of Cu(II) from aqueous solution by anatase mesoporous TiO2 nanofibers prepared via electrospinning. J. Colloid Interface Sci. 2012, 367, 429–435. [Google Scholar] [CrossRef]
- Lakhdhar, I.; Belosinschi, D.; Mangin, P.; Chabot, B. Development of a bio-based sorbent media for the removal of nickel ions from aqueous solutions. J. Environ. Chem. Eng. 2016, 4, 3159–3169. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.X.; Cao, L.X.; Yang, C.F. Enhanced chromium (VI) adsorption using nanosized chitosan fibers tailored by electrospinning. Carbohydr. Polym. 2015, 125, 206–213. [Google Scholar] [CrossRef]
- Haider, S.; Park, S.Y. Preparation of the electrospun chitosan nanofibers and their applications to the adsorption of Cu(II) and Pb(II) ions from an aqueous solution. J. Membr. Sci. 2009, 328, 90–96. [Google Scholar] [CrossRef]
- Christos, C.; Katerina, P.; Theodora, K.C.; Ioannis, P. Uranium adsorption by polyvinylpyrrolidone/chitosan blended nanofibers. Carbohydr. Polym. 2019, 219, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Lipatova, I.; Makarova, L.; Yusova, A.A. Adsorption removal of anionic dyes from aqueous solutions by chitosan nanoparticles deposited on the fibrous carrier. Chemosphere 2018, 212, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.; Li, X.; Wang, C. Adsorption of As(III) from aqueous solution based on porous magnetic/chitosan/ferric hydroxide microspheres prepared via electrospraying. Sci. Chin. Chem. 2013, 56, 678–684. [Google Scholar] [CrossRef]
- Wang, P.; Wang, L.; Dong, S.; Zhang, G.; Shi, X.; Xiang, C.; Li, L. Adsorption of hexavalent chromium by novel chitosan/poly (ethylene oxide)/permutit electrospun nanofibers. New J. Chem. 2018, 42, 17740–17749. [Google Scholar] [CrossRef]
- Tu, H.; Huang, M.; Yi, Y.; Li, Z.S.; Zhan, Y.F.; Chen, J.J.; Wu, Y.; Shi, X.W.; Deng, H.B.; Du, Y.M. Chitosan-rectorite nanospheres immobilized on polystyrene fibrous mats via alternate electrospraying/electrospinning techniques for copper ions adsorption. Appl. Surf. Sci. 2017, 426, 545–553. [Google Scholar] [CrossRef]
- Sangsanoh, P.; Supaphol, P. Stability improvement of electrospun chitosan nanofibrous membranes in neutral or weak basic aqueous solutions. Biomacromolecules 2006, 7, 2710–2714. [Google Scholar] [CrossRef]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Wu, J.; Ding, Y.; Wang, J. Facile fabrication of nanofibers- and micro/nanospheres-coordinated PVDF membrane with ultrahigh permeability of viscous water-in-oil emulsions. J. Mater. Chem. A 2018, 10, 1039–1049. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, S.; Zhao, K.; Teng, L.T.; Xie, G.W. Fabrication of TiO2 micro-/nano-spheres embedded in nanofibers by coaxial electrospinning. Mater. Res. Bull. 2016, 78, 11–15. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Q.; Yang, Q.; Li, Y.C.; Wang, W.; Sun, L.; Zhang, C.Q.; Li, Y.X. Electrospun poly (methyl methacrylate) nanofibers and microparticles. J. Mater. Sci. 2010, 45, 1032–1038. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Wen, L.; Yang, C.; Zhang, L.J. A multi-functional-group modified cellulose for enhanced heavy metal cadmium adsorption: Performance and quantum chemical mechanism. Chemosphere 2019, 224, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Kim, K.; Wang, X.; Fang, D.F.; Hsiao, B.S.; Chu, B. High flux ultrafiltration membranes based on electrospun nanofibrous PAN scaffolds and chitosan coating. Polymer 2006, 47, 2434–2441. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, W.; Yue, Y.; Xuan, L.; Ni, X.; Han, G. Electrospun cellulose nanocrystals/chitosan/polyvinyl alcohol nanofibrous films and their exploration to metal ions adsorption. Polymers 2018, 10, 1046. [Google Scholar] [CrossRef] [PubMed]
- Saeed, K.; Haider, S.; Oh, T.J.; Park, S.Y. Preparation of amidoxime-modified polyacrylonitrile (PAN-oxime) nanofibers and their applications to metal ions adsorption. J. Membr. Sci. 2008, 322, 400–405. [Google Scholar] [CrossRef]
- Pan, K.; Chang, J.; Wang, J. Preparation of α-Fe2O3/polyacrylonitrile nanofiber mat as effective lead adsorbent. Environ. Sci. 2016, 10, 1039. [Google Scholar]
- Wan, W.S.; Fatinathan, S. Adsorption characterization of Pb(II) and Cu(II) ions onto chitosan-tripolyphosphate beads: Kinetic, equilibrium and thermodynamic studies. J. Environ. Manag. 2010, 91, 958–969. [Google Scholar]
- Shariful, M.I.; Sepehr, T.; Mehrali, M.; Ang, B.C.; Amal, M.A. Adsorption capability of heavy metals by chitosan/poly (ethylene oxide)/activated carbon electrospun nanofibrous membrane. J. Appl. Polym. Sci. 2017, 135, 45851. [Google Scholar] [CrossRef]
- Wu, R.X.; Zheng, G.F.; Li, W.W.; Zhong, L.B.; Zheng, Y.M. Electrospun chitosan nanofiber membrane for adsorption of Cu(II) from aqueous solution: Fabrication, characterization and performance. J. Nanosci. Nanotechnol. 2018, 18, 5624–5635. [Google Scholar] [CrossRef]
- Wsw, N.; Endud, C.S.; Mayanar, R. Removal of copper (II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar]
- Haider, S.; Ali, F.A.A.; Haider, A.; Al-Masry, W.A. Novel route for amine grafting to chitosan electrospun nanofibers membrane for the removal of copper and lead ions from aqueous medium. Carbohydr. Polym. 2018, 199, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bai, R. Adsorptive removal of copper ions with highly porous chitosan/cellulose acetate blend hollow fiber membranes. J. Membr. Sci. 2006, 284, 313–322. [Google Scholar] [CrossRef]
- Caedenas, A.M.; Miranda, G.; Patricia, S. FTIR and TGA studies of chitosan composite films. J. Chil. Chem. Soc. 2004, 49, 291–295. [Google Scholar]














| Sample | Viscosity (Pa·s) | Electrical Conductivity (μS cm−1) | |
|---|---|---|---|
| CS-F | HFIP/FA = 9/1 | 0.722 | 2.550 |
| HFIP/FA = 6/1 | 0.856 | 3.590 | |
| HFIP/FA = 3/1 | 0.975 | 5.920 | |
| CS-M | 2 wt% CS | 0.288 | 2.530 |
| 3 wt% CS | 1.315 | 3.350 | |
| 4 wt% CS | 4.680 | 4.510 | |
| Sample | Surface Area (m2/g) | Pore Volume (cc/g) | Pore Diameter (nm) |
|---|---|---|---|
| CS-L1 | 18.7 | 0.023 | 2.65 |
| CS-L3 | 24.3 | 0.043 | 3.40 |
| CS-L5 | 25.1 | 0.047 | 3.79 |
| Sample | qe, exp (mg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| k1 | R2 | qe, cal (mg/g) | k2 | R2 | qe, cal (mg/g) | ||
| CS-L1 | 174.9 | 0.0113 | 0.8781 | 36.7 | 1.03 × 10−3 | 0.9991 | 178.8 |
| CS-L3 | 185.4 | 0.0104 | 0.7844 | 44.0 | 1.37 × 10−3 | 0.9999 | 186.9 |
| Sample | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| KL | qm (mg/g) | R2 | KF | n | R2 | |
| CS-L1 | 7.38 × 10−3 | 232.6 | 0.9951 | 5.94 | 1.89 | 0.9369 |
| CS-L3 | 2.80 × 10−2 | 276.2 | 0.9924 | 26.14 | 2.81 | 0.9717 |
| Adsorbent | qm (mg/g) | Ref. |
|---|---|---|
| PS fibrous with CS-rectorite nanospheres | 134.0 | [26] |
| CS/PEO/activated carbon nanofibers | 195.3 | [38] |
| CS/PVA nanofibers | 90.3 | [39] |
| CS beads | 80.7 | [40] |
| Amine grafted CS nanofibers | 166.7 | [41] |
| CS-L3 membranes | 276.2 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Shi, X.; Ma, L.; Pang, X.; Li, L. Preparation of Chitosan Stacking Membranes for Adsorption of Copper Ions. Polymers 2019, 11, 1463. https://doi.org/10.3390/polym11091463
Zhang X, Shi X, Ma L, Pang X, Li L. Preparation of Chitosan Stacking Membranes for Adsorption of Copper Ions. Polymers. 2019; 11(9):1463. https://doi.org/10.3390/polym11091463
Chicago/Turabian StyleZhang, Xiaoxiao, Xuejuan Shi, Liang Ma, Xuan Pang, and Lili Li. 2019. "Preparation of Chitosan Stacking Membranes for Adsorption of Copper Ions" Polymers 11, no. 9: 1463. https://doi.org/10.3390/polym11091463