Highly Selective Fluorescence Sensing and Imaging of ATP Using a Boronic Acid Groups-Bearing Polythiophene Derivate
Abstract
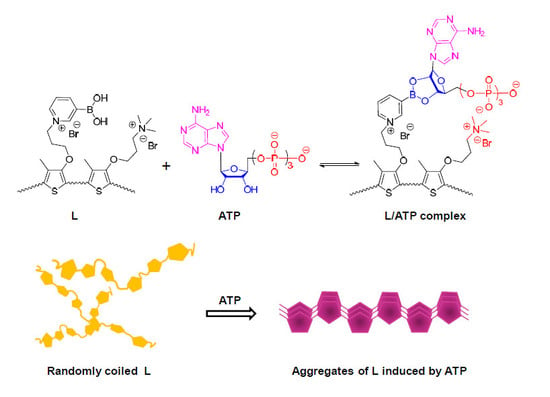
:1. Introduction
2. Experimental
2.1. Materials
2.2. Instruments
2.3. Synthesis
2.3.1. Synthesis of 3-(3-Bromopropoxy)-4-methylthiophene (1)
2.3.2. Synthesis of 3-(4-Methyl-3′-thienyloxy)propyltrimethylammonium Bromide (2)
2.3.3. Synthesis of Copolymer (3)
2.3.4. Synthesis of Copolymer L
2.4. Sample Preparation for Spectroscopic Analysis
2.5. Cell Culture and Cytotoxicity Assays
2.6. Fluorescence Imaging
3. Results and Discussion
3.1. Synthesis of L
3.2. Screen of pH of Assay System
3.3. Selectivity
3.4. UV-Vis Spectral Response of Probe L to ATP
3.5. Fluorescence Spectral Response of Probe L to ATP
3.6. Assay for ADK
3.7. Cell Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abraham, E.H.; Okunieff, P.; Scala, S.; Vos, P.; Oosterveld, M.J.S.; Chen, A.Y.; Shrivastav, B.; Guidotti, G. Cystic fibrosis transmembrane conductance regulator and adenosine triphosphate. Science 1997, 275, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.A.; Zahs, K.R. Calcium waves in retinal glial cells. Science 1997, 275, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, A.; Piku̵a, S.A. Adenosine 5′-triphosphate: An intracellular metabolic messenger. BBA Bioenerg. 1998, 1365, 333–353. [Google Scholar] [CrossRef]
- Eguchi, Y.; Shimizu, S.; Tsujimoto, Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997, 57, 1835–1840. [Google Scholar] [PubMed]
- Chen, J.; Liu, Y.; Ji, X.; He, Z. Target-protecting dumbbell molecular probe against exonucleases digestion for sensitive detection of ATP and streptavidin. Biosens. Bioelectron. 2016, 83, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, C.; Tang, L.; Qin, A.; Hu, R.; Sun, J.Z.; Tang, B.Z. Specific detection of D-glucose by a tetraphenylethene-based fluorescent sensor. J. Am. Chem. Soc. 2011, 133, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.T.; Keller, S.H.; Nigam, S.K. Genesis and reversal of the ischemic phenotype in epithelial cells. J. Clin. Investig. 2000, 106, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Zhao, M. In-vivo fluorescence imaging of adenosine 5′-triphosphate. TRAC Trend. Anal. Chem. 2016, 80, 190–203. [Google Scholar] [CrossRef]
- Quang, D.T.; Kim, J.S. Fluoro- and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem. Rev. 2010, 110, 6280–6301. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.; Yoon, J. Fluorescent and colorimetric chemosensors for detection of nucleotides, FAD and NADH: Highlighted research during 2004–2010. Chem. Soc. Rev. 2011, 40, 2222–2235. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem. Soc. Rev. 2013, 42, 622–661. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; He, W.; Guo, Z. Metal coordination in photoluminescent sensing. Chem. Soc. Rev. 2013, 42, 1568–1600. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem. Rev. 2014, 114, 590–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, J.; Yoon, J. Recent advances in development of chiral fluorescent and colorimetric sensors. Chem. Rev. 2014, 114, 4918–4959. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Bazan, G.C.; Liu, B. Conjugated-polymer-amplified sensing, imaging, and therapy. Chem 2017, 2, 760–790. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Water-soluble conjugated polymers for imaging, diagnosis, and therapy. Chem. Rev. 2012, 112, 4687–4735. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Qiu, T.; Liu, L.; Ying, J.; Wang, S. Recent advances in conjugated polymer materials for disease diagnosis. Small 2016, 12, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Li, Y.; Wang, J.; Sun, Y.; Jin, L.; Li, C.; Lu, Y. Fluorescence and colorimetric detection of ATP based on a strategy of self-promoting aggregation of a water-soluble polythiophene derivative. Chem. Commun. 2015, 51, 8544–8546. [Google Scholar] [CrossRef]
- Li, C.; Numata, M.; Takeuchi, M.; Shinkai, S. A sensitive colorimetric and fluorescent probe based on a polythiophene derivative for the detection of ATP. Angew. Chem. Int. Ed. 2005, 44, 6371–6374. [Google Scholar] [CrossRef]
- Lin, C.; Cai, Z.; Wang, Y.; Zhu, Z.; Yang, C.J.; Chen, X. Label-free fluorescence strategy for sensitive detection of adenosine triphosphate using a loop DNA probe with low background noise. Anal. Chem. 2014, 86, 6758–6762. [Google Scholar] [CrossRef]
- Lu, C.H.; Li, J.; Lin, M.H.; Wang, Y.W.; Yang, H.H.; Chen, X.; Chen, G.N. Amplified aptamer-based assay through catalytic recycling of the analyte. Angew. Chem. Int. Ed. 2010, 49, 8454–8457. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.H.; Geng, Z.R.; Ma, X.Y.; Zhang, C.; Zhang, Z.Y.; Wang, Z.L. Lysosomal ATP imaging in living cells by a water-soluble cationic polythiophene derivative. Biosens. Bioelectron. 2016, 83, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Geng, Z.; Yan, S.; Li, Z.; Cai, J.; Wang, Z. Water-soluble conjugated polymer as a fluorescent probe for monitoring adenosine triphosphate level fluctuation in cell membranes during cell apoptosis and in vivo. Anal. Chem. 2017, 89, 8816–8821. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Zhang, Q.; Wang, J.; Liu, C.; Shi, L.; Liu, L.; Deng, L.; Lu, Y. A new FRET-based ratiometric probe for fluorescence and colorimetric analyses of adenosine 5′-triphosphate. Polym. Chem. 2017, 8, 1138–1145. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; An, N.; Wang, J.; Zhao, L.; Lu, Y. A new water-soluble polythiophene derivative as a probe for real-time monitoring adenosine 5′-triphosphatase activity in lysosome of living cells. Talanta 2018, 182, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Cai, F.; Liu, H. Ultrasensitive fluorescent responses of water-soluble, zwitterionic, boronic acid-bearing, regioregular head-to-tail polythiophene to biological species. Chem.-Eur. J. 2008, 14, 1648–1653. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.M.; Lee, M.; Yoon, J. Polydiacetylenes bearing boronic acid groups as colorimetric and fluorescence sensors for cationic surfactants. ACS Appl. Mater. Inter. 2013, 5, 4521–4526. [Google Scholar] [CrossRef]
- DiCesare, N.; Lakowicz, J.R. Spectral properties of fluorophores combining the boronic acid group with electron donor or withdrawing groups. Implication in the development of fluorescence probes for saccharides. J. Phys. Chem. A 2001, 105, 6834–6840. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Wang, B. New boronic acid fluorescent reporter compounds. 2. A naphthalene-based on-off sensor functional at physiological pH. Org. Lett. 2003, 5, 4615–4618. [Google Scholar] [CrossRef]
- Mohler, L.K.; Czarnik, A.W. Ribonucleoside membrane transport by a new class of synthetic carrier. J. Am. Chem. Soc. 1993, 115, 2998–2999. [Google Scholar] [CrossRef]
- Li, C.; Shi, G. Polythiophene-based optical sensors for small molecules. ACS Appl. Mater. Inter. 2013, 5, 4503–4510. [Google Scholar] [CrossRef] [PubMed]
- Spangler, C.; Lang, T.; Schäferling, M. Spectral response of a methylimidazolium-functionalized polythiophene to phosphates. Dyes Pigm. 2012, 95, 194–200. [Google Scholar] [CrossRef]
- Stephanos, J.J. Drug-protein interactions: Two-site binding of heterocyclic ligands to a monomeric hemoglobin. J. Inorg. Biochem. 1996, 62, 155–169. [Google Scholar] [CrossRef]
- Froehlich, E.; Mandeville, J.S.; Weinert, C.M.; Kreplak, L.; Tajmir-Riahi, H.A. Bundling and aggregation of DNA by cationic dendrimers. Biomacromolecules 2011, 12, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Lu, Y.; Zhang, Q.; Sun, J.; Zeng, X.; Li, C. Homogeneous and sensitive DNA detection based on polyelectrolyte complexes of cationic conjugated poly(pyridinium salt)s and DNA. J. Mater. Chem. 2012, 22, 4106–4112. [Google Scholar] [CrossRef]
- Li, C.; Numata, M.; Takeuchi, M.; Shinkai, S. Unexpected chiroptical inversion observed for supramolecular complexes formed between an achiral polythiophene and ATP. Chem.-Asian J. 2006, 1, 95–101. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Introduction to Fluorescence. In Principles of Fluorescence Spectroscopy, 3rd ed.; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2006; pp. 1–26. [Google Scholar]
- Caballero, A.; Martínez, R.; Lloveras, V.; Ratera, I.; Vidal-Gancedo, J.; Wurst, K.; Tárraga, A.; Molina, P.; Veciana, J. Highly selective chromogenic and redox or fluorescent sensors of Hg2+ in aqueous environment based on 1,4-disubstituted azines. J. Am. Chem. Soc. 2005, 127, 15666–15667. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhao, L.; Cheng, D.; Yao, X.; Lu, Y. Highly Selective Fluorescence Sensing and Imaging of ATP Using a Boronic Acid Groups-Bearing Polythiophene Derivate. Polymers 2019, 11, 1139. https://doi.org/10.3390/polym11071139
Liu L, Zhao L, Cheng D, Yao X, Lu Y. Highly Selective Fluorescence Sensing and Imaging of ATP Using a Boronic Acid Groups-Bearing Polythiophene Derivate. Polymers. 2019; 11(7):1139. https://doi.org/10.3390/polym11071139
Chicago/Turabian StyleLiu, Lihua, Linlin Zhao, Dandan Cheng, Xinyi Yao, and Yan Lu. 2019. "Highly Selective Fluorescence Sensing and Imaging of ATP Using a Boronic Acid Groups-Bearing Polythiophene Derivate" Polymers 11, no. 7: 1139. https://doi.org/10.3390/polym11071139