Probing Chemical Changes in Holocellulose and Lignin of Timbers in Ancient Buildings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Lignin, Cellulose, and Hemicellulose
2.3. Characterizations
3. Results and Discussion
3.1. Determination of Lignin, Cellulose, and Hemicellulose
3.2. FTIR Analysis
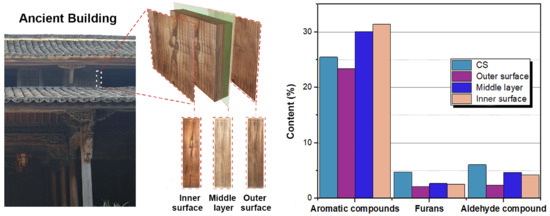
3.3. Py-GC/MS
3.4. Elemental Analysis
3.5. XRD
3.6. Thermal Analysis
3.7. Morphology Observation
3.8. XPS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colombini, M.P.; Lucejko, J.J.; Modugno, F.; Orlandi, M.; Tolppa, E.-L.; Zoia, L. A multi-analytical study of degradation of lignin in archaeological waterlogged wood. Talanta 2009, 80, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Inari, G.N.; Petrissans, M.; Lambert, J.; Ehrhardt, J.J.; Gérardin, P. XPS characterization of wood chemical composition after heat-treatment. Surf. Interface Anal. 2006, 38, 1336–1342. [Google Scholar] [CrossRef] [Green Version]
- Popescu, C.-M.; Dobele, G.; Rossinskaja, G.; Dizhbite, T.; Vasile, C. Degradation of lime wood painting supports. J. Anal. Appl. Pyrolysis 2007, 79, 71–77. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Hill, C.A.S. The water vapour adsorption–desorption behaviour of naturally aged Tilia cordata Mill. wood. Polym. Degrad. Stab. 2013, 98, 1804–1813. [Google Scholar] [CrossRef]
- Bardet, M.; Foray, M.F.; Maron, S.; Goncalves, P.; Trân, Q.K. Characterization of wood components of Portuguese medieval dugout canoes with high-resolution solid-state NMR. Carbohydr. Polym. 2004, 57, 419–424. [Google Scholar] [CrossRef]
- Sandak, A.; Sandak, J.; Babiński, L.; Pauliny, D.; Riggio, M. Spectral analysis of changes to pine and oak wood natural polymers after short-term waterlogging. Polym. Degrad. Stab. 2014, 99, 68–79. [Google Scholar] [CrossRef]
- Tamburini, D.; Łucejko, J.J.; Modugno, F.; Colombini, M.P. Characterisation of archaeological waterlogged wood from Herculaneum by pyrolysis and mass spectrometry. Int. Biodeterior. Biodegrad. 2014, 86, 142–149. [Google Scholar] [CrossRef]
- Colombini, M.P.; Orlandi, M.; Modugno, F.; Tolppa, E.L.; Sardelli, M.; Zoia, L.; Crestini, C. Archaeological wood characterisation by PY/GC/MS, GC/MS, NMR and GPC techniques. Microchem. J. 2007, 85, 164–173. [Google Scholar] [CrossRef]
- Łucejko, J.J.; Modugno, F.; Ribechini, E.; del Río, J.C. Characterisation of archaeological waterlogged wood by pyrolytic and mass spectrometric techniques. Anal. Chim. Acta 2009, 654, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sáiz-Jiménez, C.; Boon, J.J.; Hedges, J.I.; Hessels JK, C.; De Leeuw, J.W. Chemical characterization of recent and buried woods by analytical pyrolysis Comparison of pyrolysis data with 13C NMR and wet chemical data. J. Anal. Appl. Pyrolysis 1987, 11, 437–450. [Google Scholar] [CrossRef]
- Vinciguerra, V.; Napoli, A.; Bistoni, A.; Petrucci, G.; Sgherzi, R. Wood decay characterization of a naturally infected London plane-tree in urban environment using Py-GC/MS. J. Anal. Appl. Pyrolysis 2007, 78, 228–231. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Yamada, T.; Capanema, E.; Chang, H.-M.; Chiang, V.; Kadla, J.F. Rapid Screening of Wood Chemical Component Variations Using Transmittance Near-Infrared Spectroscopy. J. Agric. Food Chem. 2005, 53, 3328–3332. [Google Scholar] [CrossRef] [PubMed]
- Belkhir, S.; Koubaa, A.; Khadhri, A.; Ksontini, M.; Nadji, H.; Smiti, S.; Stevanovic, T. Seasonal effect on the chemical composition of the leaves of Stipa tenacissima L. and implications for pulp properties. Ind. Crop. Prod. 2013, 44, 56–61. [Google Scholar] [CrossRef]
- Labidi, K.; Korhonen, O.; Zrida, M.; Hamzaoui, A.H.; Budtova, T. All-cellulose composites from alfa and wood fibers. Ind. Crop. Prod. 2019, 127, 135–141. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.V.; Thain, S.C.; Donnison, I.S.; Morris, P.M.; Yates, N. Prediction of Klason lignin and lignin thermal degradation products by Py–GC/MS in a collection of Lolium and Festuca grasses. J. Anal. Appl. Pyrolysis 2007, 80, 16–23. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, X.C.; Dong, C.Q.; Zhang, Z.F.; Zhang, X.M.; Zhu, X.F. Influence of pyrolysis temperature and time on the cellulose fast pyrolysis products: Analytical Py-GC/MS study. J. Anal. Appl. Pyrolysis 2011, 92, 430–438. [Google Scholar] [CrossRef]
- Lu, Q.; Li, W.Z.; Zhang, D.; Zhu, X.F. Analytical pyrolysis–gas chromatography/mass spectrometry (Py–GC/MS) of sawdust with Al/SBA-15 catalysts. J. Anal. Appl. Pyrolysis 2009, 84, 131–138. [Google Scholar] [CrossRef]
- Zhang, M.; Resende, F.L.P.; Moutsoglou, A.; Raynie, D.E. Pyrolysis of lignin extracted from prairie cordgrass, aspen, and Kraft lignin by Py-GC/MS and TGA/FTIR. J. Anal. Appl. Pyrolysis 2012, 98, 65–71. [Google Scholar] [CrossRef]
- Thygesen, A.; Oddershede, J.; Lilholt, H.; Thomsen, A.B.; Ståhl, K. On the determination of crystallinity and cellulose content in plant fibres. Cellulose 2005, 12, 563–576. [Google Scholar] [CrossRef]
- Xie, J.; Qi, J.; Huang, X.; Zhou, N.; Hu, Y. Comparative analysis of modern and ancient buried Phoebe zhennan wood: Surface color, chemical components, infrared spectroscopy, and essential oil composition. J. For. Res. 2015, 26, 501–507. [Google Scholar] [CrossRef]
- Song, P.; Dai, J.; Chen, G.; Yu, Y.; Fang, Z.; Lei, W.; Fu, S.; Wang, H.; Chen, Z.-G. Bioinspired Design of Strong, Tough, and Thermally Stable Polymeric Materials via Nanoconfinement. ACS Nano 2018, 12, 9266–9278. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Xu, Z.; Dargusch, M.S.; Chen, Z.-G.; Wang, H.; Guo, Q. Granular Nanostructure: A Facile Biomimetic Strategy for the Design of Supertough Polymeric Materials with High Ductility and Strength. Adv. Mater. 2017, 29, 1704661. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Xu, Z.; Lu, Y.; Guo, Q. Bio-Inspired Hydrogen-Bond Cross-Link Strategy toward Strong and Tough Polymeric Materials. Macromolecules 2015, 48, 3957–3964. [Google Scholar] [CrossRef]
- Song, P.A.; Xu, Z.; Guo, Q. Bioinspired Strategy to Reinforce PVA with Improved Toughness and Thermal Properties via Hydrogen-Bond Self-Assembly. ACS Macro Lett. 2013, 2, 1100–1104. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Antoine, R.; Hsiao, B.S. Efficient Removal of Arsenic Using Zinc Oxide Nanocrystal-Decorated Regenerated Microfibrillated Cellulose Scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 6140–6151. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Hsiao, B.S. Efficient Removal of UO22+ from Water Using Carboxycellulose Nanofibers Prepared by the Nitro-Oxidation Method. Ind. Eng. Chem. Res. 2017, 56, 13885–13893. [Google Scholar] [CrossRef]
- Hosoya, T.; Kawamoto, H.; Saka, S. Cellulose–hemicellulose and cellulose–lignin interactions in wood pyrolysis at gasification temperature. J. Anal. Appl. Pyrolysis 2007, 80, 118–125. [Google Scholar] [CrossRef]
- Hosoya, T.; Kawamoto, H.; Saka, S. Pyrolysis behaviors of wood and its constituent polymers at gasification temperature. J. Anal. Appl. Pyrolysis 2007, 78, 328–336. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Wang, K.; Luo, Z. Influence of the interaction of components on the pyrolysis behavior of biomass. J. Anal. Appl. Pyrolysis 2011, 91, 183–189. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.-M.; Tibirna, C.-M.; Vasile, C. XPS characterization of naturally aged wood. Appl. Surf. Sci. 2009, 256, 1355–1360. [Google Scholar] [CrossRef]








| Composition | CS | LS-O | LS-M | LS-I |
|---|---|---|---|---|
| Klason lignin (wt %) | 31.37 | 30.05 | 34.00 | 34.51 |
| Holocellulose (wt %) | 66.85 | 56.65 | 63.33 | 59.42 |
| Cellulose (wt %) | 46.53 | 41.87 | 45.29 | 43.63 |
| Hemicellulose (wt %) | 20.32 | 14.78 | 18.04 | 15.79 |
| Sample | C (wt %) | H (wt %) | O 1 (wt %) | N (wt %) | S (wt %) |
|---|---|---|---|---|---|
| CS | 48.79 | 6.90 | 44.29 | 0 | 0.018 |
| LS-M | 47.83 | 6.75 | 44.62 | 0.64 | 0.161 |
| LS-I | 47.52 | 6.67 | 45.10 | 0.62 | 0.094 |
| LS-O | 47.24 | 6.53 | 45.31 | 0.76 | 0.164 |
| Sample Number | CS | LS-O | LS-M | LS-I |
|---|---|---|---|---|
| Degree of crystallinity (%) | 35.89 | 32.04 | 35.81 | 34.62 |
| Sample | Peak Area Percentage (%) | ||
|---|---|---|---|
| C1 (C–C) | C2 (C–O) | C3 (C=O) | |
| CS | 34 | 50 | 16 |
| LS-O | 31 | 35 | 34 |
| LS-M | 33 | 40 | 27 |
| LS-I | 38 | 30 | 32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Zhang, X.; Liu, L.; Yu, Y.; Zheng, W.; Song, P. Probing Chemical Changes in Holocellulose and Lignin of Timbers in Ancient Buildings. Polymers 2019, 11, 809. https://doi.org/10.3390/polym11050809
Zhao C, Zhang X, Liu L, Yu Y, Zheng W, Song P. Probing Chemical Changes in Holocellulose and Lignin of Timbers in Ancient Buildings. Polymers. 2019; 11(5):809. https://doi.org/10.3390/polym11050809
Chicago/Turabian StyleZhao, Chencheng, Xiaochun Zhang, Lina Liu, Youming Yu, Wei Zheng, and Pingan Song. 2019. "Probing Chemical Changes in Holocellulose and Lignin of Timbers in Ancient Buildings" Polymers 11, no. 5: 809. https://doi.org/10.3390/polym11050809