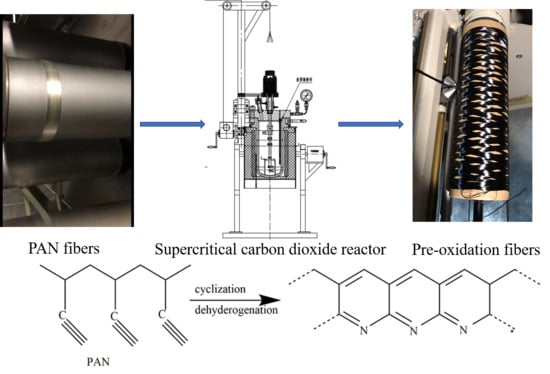
Study on the Changes of Structures and Properties of PAN Fibers during the Cyclic Reaction in Supercritical Carbon Dioxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Characterizations
3. Results and Discussion
3.1. FT-IR Evolution Analysis of the PAN Fibers Heated at Different Temperatures
3.2. X-ray Diffraction Analysis of PAN Fibers Heated at Different Temperatures
3.3. Differential Scanning Calorimeter Analysis of the PAN Fibers Heated at Different Temperatures
3.4. Thermogravimetric Analysis of PAN Polymers Heated at Different Temperatures
3.5. Density Changes of PAN Fibers Treated in Different Conditions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Park, S.-J. History and Structure of Carbon Fibers; Springer Nature: Heidelberg, Germany, 2018; pp. 1–30. [Google Scholar]
- Park, S.J.; Kim, B.J. Carbon Fibers and Their Composites; Springer Netherlands: Berlin, Germany, 2015; pp. 275–317. [Google Scholar]
- Li, H.Y.; Dai, Y.; Lyu, X.F. Application of Carbon Fiber Reinforced Composites on Lightweight Design of Articulated Platform. MSF 2018, 921, 85–90. [Google Scholar] [CrossRef]
- Park, S.-J. Novel Carbon Fibers and Their Composites; Springer Nature: Heidelberg, Germany, 2018; Volume 210, pp. 295–342. [Google Scholar]
- Peng, M.; Ma, J. Carbon fiber and its application in light weighting of automobiles. China Synth. Fiber Ind. 2018, 41, 53–57. [Google Scholar]
- Wang, Y.Z.; Wang, S.G.; Liu, J.L. Polyacrylonitrile Based Carbon Fibers Obtained from a Melt Spun Route. KEM 2013, 575, 151–155. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, J.; Liu, L.; Dong, Y.; Liu, A. Mesophase pitch-based carbon fiber spinning through a filter assembly and the microstructure evolution mechanism. J. Mater. Sci. 2013, 49, 191–198. [Google Scholar] [CrossRef]
- Niu, H.; Chang, J.; Liu, W.; Yang, H.; Cao, L.; Zhang, M.; Cao, W.; Wu, D. Structural relationship between random copolyimides and their carbon fibers. J. Mater. Sci. 2016, 52, 1883–1897. [Google Scholar]
- Behr, M.J.; Landes, B.G.; Barton, B.E.; Bernius, M.T.; Billovits, G.F.; Hukkanen, E.J.; Patton, J.T.; Wang, W.; Wood, C.; Keane, D.T.; et al. Structure-property model for polyethylene-derived carbon fiber. Carbon 2016, 107, 525–535. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Amp, S. Technical Advances and Development Suggestions for Rayon-based Carbon Fiber and Pitch-based Carbon Fiber. Chem. Fert. Des. 2017, 55, 1–3. [Google Scholar]
- Frank, E.; Hermanutz, F.; Buchmeiser, M.R. Carbon Fibers: Precursors, Manufacturing, and Properties. Macromol. Mater. Eng. 2012, 297, 493–501. [Google Scholar] [CrossRef]
- Aso, H.; Kannabe, T. Polyacrylonitrile-based carbon fiber. TANSO 2007, 2007, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.S.; Ogale, A.A. Carbon fibers derived from UV-assisted stabilization of wet-spun polyacrylonitrile fibers. J. Appl. Polym. Sci. 2014, 131, 318–323. [Google Scholar] [CrossRef]
- Zhao, W.; Lu, Y.; Wang, J.; Chen, Q.; Zhou, L.; Jiang, J.; Chen, L. Improving crosslinking of stabilized polyacrylonitrile fibers and mechanical properties of carbon fibers by irradiating with γ-ray. Polym. Degrad. Stab. 2016, 133, 16–26. [Google Scholar] [CrossRef]
- Zhao, R.-X.; Sun, P.-F.; Liu, R.-J.; Ding, Z.-H.; Li, X.-S.; Liu, X.-Y.; Zhao, X.-D.; Gao, Z.-M. Influence of heating procedures on the surface structure of stabilized polyacrylonitrile fibers. Appl. Surf. Sci. 2018, 433, 321–328. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, S.; Shen, Z.; Xu, L.; Zhang, L.; Peng, J. Study on the oxidative stabilization of polyacrylonitrile fibers by microwave heating. Polym. Degrad. Stab. 2018, 150, 86–91. [Google Scholar] [CrossRef]
- Fitzer, E.; Frohs, W.; Heine, M. Optimization of stabilization and carbonization treatment of PAN fibres and structural characterization of the resulting carbon fibres. Carbon 1986, 24, 387–395. [Google Scholar] [CrossRef]
- Banerjee, T.; Lipscomb, G.G. Direct measurement of the carbon dioxide-induced glass transition depression in a family of substituted polycarbonates. J. Appl. Polym. Sci. 1998, 68, 1441–1449. [Google Scholar] [CrossRef]
- Elmaaty, T.A.; El-Taweel, F.; Okubayashi, S.; Ma, J.; El-Aziz, E.A. Facile Bifunctional Dyeing of Polyester under Supercritical Carbon Dioxide Medium with New Antibacterial Hydrazono Propanenitrile Dyes. Ind. Eng. Chem. Res. 2014, 53, 15566–15570. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, J.; Yan, J.; Zheng, L. An industrial scale multiple supercritical carbon dioxide apparatus and its eco-friendly dyeing production. J. CO2 Util. 2016, 16, 272–281. [Google Scholar] [CrossRef]
- Zheng, H.-D.; Zhang, J.; Yan, J.; Zheng, L.-J. Investigations on the effect of carriers on meta-aramid fabric dyeing properties in supercritical carbon dioxide. RSC Adv. 2017, 7, 3470–3479. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, J.P.; Pryde, E.H. Supercritical CO2 extraction of lipid-bearing materials and characterization of the products. J. Am. Oil Chem. Soc. 1984, 61, 223–228. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Rodríguez-Perrino, M.; Sanz, M.T.; Melgosa, R.; Beltran, S. Supercritical carbon dioxide extraction of quinoa oil: Study of the influence of process parameters on the extraction yield and oil quality. J. Supercrit. Fluids 2018, 139, 62–71. [Google Scholar]
- Bonthuys, G.J.K.; Schwarz, C.E.; Burger, A.J.; Knoetze, J.H. Separation of alkanes and alcohols with supercritical fluids. Part I: Phase equilibria and viability study. J. Supercrit. Fluids 2011, 57, 101–111. [Google Scholar] [CrossRef]
- Clifford, A.A. Reactions in Supercritical Fluids; Springer Netherlands: Berlin, Germany, 1994; pp. 449–479. [Google Scholar]
- Dudnik, A.O.; Trofimchuk, E.S.; Efimov, A.V.; Nikonorova, N.I.; Rukhlya, E.G.; Nikitin, L.N.; Yaminsky, I.V.; Volynskii, A.L. Evolution of the Nanoporous Structure of High-Density Polyethylene during Drawing in Supercritical Carbon Dioxide. Macromolecules 2018, 51, 1129–1140. [Google Scholar] [CrossRef]
- Rebocho, S.; Cordas, C.M.; Viveiros, R.; Casimiro, T. Development of a ferrocenyl-based MIP in supercritical carbon dioxide: Towards an electrochemical sensor for bisphenol A. J. Supercrit. Fluids 2018, 135, 98–104. [Google Scholar] [CrossRef]
- Johns, A.I.; Rashid, S.; Watson, J.T.R.; Clifford, A.A. Thermal conductivity of argon, nitrogen and carbon dioxide at elevated temperatures and pressures. J. Chem. Soc. Faraday Trans. 1 1986, 82, 2235. [Google Scholar] [CrossRef]
- Kouta, A.; Al-Sulaiman, F.; Atif, M.; Bin Marshad, S. Entropy, exergy, and cost analyses of solar driven cogeneration systems using supercritical CO2 Brayton cycles and MEE-TVC desalination system. Energy Convers. Manag. 2016, 115, 253–264. [Google Scholar] [CrossRef]
- Sengers, J.; Michels, A.; Van Der Gulik, P. The thermal conductivity of carbon dioxide in the critical region: I. The thermal conductivity apparatus. Physica 1962, 28, 1201–1215. [Google Scholar]
- Le Neindre, B. Contribution a l’etude experimentale de la conductivite thermique de quelques fluides a haute temperature et a haute pression. Int. J. Heat Mass Transf. 1972, 15, 1–24. [Google Scholar] [CrossRef]
- Stephan, K.; Laesecke, A. The Thermal Conductivity of Fluid Air. J. Phys. Chem. Ref. Data 1985, 14, 227. [Google Scholar] [CrossRef]
- Lin, X.; Wang, C.G.; Yu, M.J.; Lin, Z.T.; Zhang, S. Study on the Local Structure of PAN-Based Carbon Fiber Using Radial Distribution Function Based on XRD. AMR 2013, 664, 614–619. [Google Scholar] [CrossRef]












| Medium | Reference | T(K) | P(MPa) | λ (mW·m−1·K−1) |
|---|---|---|---|---|
| Sc-CO2 | Johns et al. [28] | 381.07–473.38 | 8.32–30.6 | 30.98–61.57 |
| Le Neindre et al. [29] | 311.25–960.85 | 7.6–127.8 | 27.7–166.0 | |
| Michels et al. [30] | 313.2–348.29 | 8.437–209.68 | 30–194.81 | |
| Le Neindre [31] | 326.65–951.15 | 10–120 | 30.8–154.4 | |
| Air | Stephan et al. [32] | 373.0–573.0 | / | 9.359–26.35 |
| Different Treatment Conditions | Residual Mass (%)/air | Residual Mass (%)/Sc-CO2 |
|---|---|---|
| Untreated fibers | 33.54 | 33.54 |
| 140 °C | 34.78 | 43.32 |
| 160 °C | 43.11 | 47.43 |
| 180 °C | 44.43 | 55.21 |
| 200 °C | 46.21 | 55.80 |
| 220 °C | 51.86 | 62.07 |
| 240 °C | 61.36 | 63.45 |
| 260 °C | 61.59 | 66.34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, M.; Kong, H.; Ding, X.; Hu, Z.; Zhang, L.; Cao, Y.; Yu, M. Study on the Changes of Structures and Properties of PAN Fibers during the Cyclic Reaction in Supercritical Carbon Dioxide. Polymers 2019, 11, 402. https://doi.org/10.3390/polym11030402
Qiao M, Kong H, Ding X, Hu Z, Zhang L, Cao Y, Yu M. Study on the Changes of Structures and Properties of PAN Fibers during the Cyclic Reaction in Supercritical Carbon Dioxide. Polymers. 2019; 11(3):402. https://doi.org/10.3390/polym11030402
Chicago/Turabian StyleQiao, Mengmeng, Haijuan Kong, Xiaoma Ding, Zhifeng Hu, Luwei Zhang, Yuanzhi Cao, and Muhuo Yu. 2019. "Study on the Changes of Structures and Properties of PAN Fibers during the Cyclic Reaction in Supercritical Carbon Dioxide" Polymers 11, no. 3: 402. https://doi.org/10.3390/polym11030402