Study on Demulsification-Flocculation Mechanism of Oil-Water Emulsion in Produced Water from Alkali/Surfactant/Polymer Flooding
Abstract
:1. Introduction
2. Materials and Experimental Methods
2.1. Experimental Materials and Instruments
2.2. Experimental Procedure
2.3. Experimental Methods
3. Results
3.1. The Effect of Demulsifier on Interfacial Tension
3.2. The Effect of Demulsifier on Zeta Potential
3.3. The Effect of Demulsifier on the Size of Oil Droplets
3.4. The Effect of Flocculant on Interfacial Tension
3.5. The Effect of Flocculant on Zeta Potential
3.6. The Effect of Flocculant on the Size of Oil Droplets
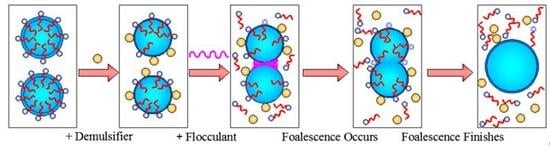
4. Demulsification-Flocculation Mechanism of Oil-Water Emulsion
5. Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhong, H.; Li, Y.; Zhang, W.; Yin, H.; Lu, J.; Guo, D. Microflow mechanism of oil displacement by viscoelastic hydrophobically associating water-soluble polymers in enhanced oil recovery. Polymers 2018, 10, 628. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, J.; Shi, F. Evaluation of the Effect of Microbial Combination Flooding. Adv. Petrol. Explor. Dev. 2016, 11, 2. [Google Scholar]
- Wei, B.; Li, Q.; Jin, F.; Li, H.; Wang, C. The Potential of a Novel Nano-fluid in Enhancing Oil Recovery. Energy Fuels 2016, 30, 2882–2891. [Google Scholar] [CrossRef]
- Wei, B.; Li, H.; Li, Q.; Lu, L.; Li, Y.; Pu, W.; Wen, Y. Investigation of Synergism between Surface-grafted Nano-cellulose and Surfactants in Stabilized Foam Injection Process. Fuel 2017, 207, 352–364. [Google Scholar] [CrossRef]
- Nedjhioui, M.; Moulai-Mostefa, N.; Morsli, A. Combined effects of polymer/surfactant/oil/alkali on physical chemical properties. Desalination 2005, 185, 543–550. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.H.; Zhuge, X.L. An environmentally-friendly method for disposal of the ASP flooding produced water. In Proceedings of the SPE Arctic and Extreme Environments Technical Conference and Exhibition, Moscow, Russia, 15–17 October 2013. [Google Scholar]
- Nguyen, D.; Sadeghi, N. Stable emulsion and demulsification in chemical EOR flooding: Challenges and best practices. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012. [Google Scholar]
- Yang, X.; Liao, G.Z.; Han, P.H.; Yang, Z.Y.; Yao, Y.N. An Extended field Test Study on Alkaline-Surfactant-Polymer Flooding in Beiyiduanxi of Daqing Oilfield. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 9–11 September 2003. [Google Scholar]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Inter. J. Low-Carbon Technol. 2014, 9, 157–177. [Google Scholar] [CrossRef]
- Zhang, X.J.; Ji, W.; Wang, B.H. Study on the treatment technology of oilfield produced water. Indust. Safety Environ. Protec. 2007, 33, 13–16. [Google Scholar]
- Li, J.X.; Liu, Y.; Wu, D.; Meng, X.C.; Zhao, F.L. Synergistic Effects of Alkaline, Surfactant, and Polymer on the Emulsification and Destabilization of Oil-in-Water Crude Oil Emulsion Produced by Alkaline-Surfactant-Polymer Flooding. Petrol. Sci. Technol. 2013, 31, 399–407. [Google Scholar] [CrossRef]
- Kudaibergenov, S.; Akhmedzhanov, T.K.; Zhappasbayev, B.Z.; Gussenov, I.S.; Shakhvorostov, A.V. Laboratory Study of ASP Flooding for Viscous Oil. Inter. J. Chem. Sci. 2015, 13, 2017–2025. [Google Scholar]
- Khambharatana, F.; Thomas, S.; Ali, S. Macroemulsion Rheology and Drop Capture Mechanism During Flow in Porous Media. In Proceedings of the SPE International Oil and Gas Conference and Exhibition in China, Society of Petroleum Engineers, Beijing, China, 2–6 November 1998. [Google Scholar]
- Fatemeh, G.; Sohrab, Z. A Comprehensive Review on Emulsions and Emulsion Stability in Chemical and Energy Industries. Can. J. Chem. Eng. 2019, 97, 281–309. [Google Scholar]
- Wang, J.; Song, C.; Li, C. Effect of ASP composition on the emulsion-stabilizing properties of simulativeproduced liquid by ASP flooding. J. China Univ. Petrol. 2016, 40, 146–154. [Google Scholar]
- Zhang, R.; Liang, C.; Wu, D.; Deng, S. Characterization and demulsification of produced liquid from weak base ASP flooding. Colloids Surf. A 2006, 290, 164–171. [Google Scholar] [CrossRef]
- Demirbas, A.; Bamufleh, H.; Edris, G.; Alalayah, W.M. Treatment of contaminated wastewater. Petrol. Sci. Technol. 2017, 35, 883–889. [Google Scholar] [CrossRef]
- Hanafy, M.; Nabih, H. Treatment of oily wastewater using dissolved air flotation technique. Energy Sources Part A 2007, 29, 143–159. [Google Scholar] [CrossRef]
- Ran, J.C.; Liu, J.T.; Zhang, C.J.; Wang, D.Y.; Li, X.B. Experimental investigation and modeling of flotation column for treatment of oily wastewater. Inter. J. Mining Sci. Technol. 2013, 23, 665–668. [Google Scholar] [CrossRef]
- Yang, F.; Tchoukov, P.; Qiao, P.Q.; Ma, X.; Pensini, E.; Dabros, T.; Czarnecki, J.; Xu, Z. Studying demulsification mechanisms of water-in-crude oil emulsions using a modified thin liquid film technique. Colloids Surf. A 2018, 540, 215–223. [Google Scholar] [CrossRef]
- Rajak, V.K.; Singh, I.; Kumar, A.; Mandal, A. Optimization of separation of oil from oil-in-water emulsion by demulsification using different demulsifiers. Petrol. Sci. Technol. 2016, 34, 1026–1032. [Google Scholar] [CrossRef]
- Dickhout, J.M.; Moreno, Y.; Biesheuvel, P.M.; Boels, L.; Lanunertink, R.G.H. Produced water treatment by membranes: A review from a colloidal perspective. J. Colloid Interf. Sci. 2017, 487, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Rajak, V.K.; Relish, K.K.; Kumar, S.; Mandal, A. Mechanism and Kinetics of Separation of Oil from Oil-in-Water Emulsion by Air Flotation. Petrol. Sci. Technol. 2015, 33, 1861–1868. [Google Scholar] [CrossRef]
- Rajak, V.K.; Kumar, S.; Thombre, N.V.; Mandal, A. Synthesis of activated charcoal from saw-dust and characterization for adsorptive separation of oil from oil-in-water emulsion. Chem. Eng. Commun. 2018, 205, 897–913. [Google Scholar] [CrossRef]
- Foulds, B. Fixed-Media Biotechnology Provides Efficient Wastewater Treatment & Quality Effluent. Plant Eng. 2013, 6, 6–7. [Google Scholar]
- Deng, S.B.; Bai, R.B.; Chen, J.P. Effects of alkaline/surfactant/polymer on stability of oil droplets in produced water from ASP flooding. Colloids Surf. A 2002, 2002, 275–284. [Google Scholar] [CrossRef]
- Deng, S.B.; Yu, G.; Jiang, Z.P. Destabilization of oil droplets in produced water from ASP flooding. Colloids Surf. A 2005, 252, 113–119. [Google Scholar] [CrossRef]
- Wang, B.; Wu, T.; Li, Y.J. The effects of oil displacement agents on the stability of water produced from ASP (alkaline/surfactant/polymer) flooding. Colloids Surf. A 2011, 379, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.T.; Li, F.; Li, Y.J. Effective removal of emulsified oil from oily wastewater using in-situ generated metallic hydroxides from leaching solution of white mud. Chem. Eng. J. 2017, 309, 513–521. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, S.L.; Zhu, Y.B. Application of polytetrafluoroethylene (PTFE) flat membrane for the treatment of pre-treated ASP flooding produced water in a Daqing oilfield. RSC Adv. 2016, 6, 62411–62419. [Google Scholar] [CrossRef]
- Miao, L.; Li, F.; Sun, D.J. Interfacial and electrokinetic properties of asphaltenes and alkali/surfactant/polymer in produced water system. J. Petrol. Sci. Eng. 2015, 133, 18–28. [Google Scholar] [CrossRef]
- Li, X.H.; Kerstern, S.R.A.; Schuur, B. Efficiency and mechanism of demulsification of oil-in-water emulsions using ionic liquids. Energy Fuels 2016, 30, 7622–7628. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, N.; Wang, J.; Tong, J. Demulsification efficiency of heavy oil-in-water emulsion stabilized by organic alkali and compound surfactants. Oilfield Chem. 2016, 33, 338–344. [Google Scholar]
- Zhang, Z. The flocculation mechanism and treatment of oily wastewater by flocculation. Water Sci. Technol. 2017, 76, 2630–2637. [Google Scholar] [CrossRef] [PubMed]









| Drugs | Sources |
|---|---|
| NaCl | Tianjin Damao Chemical Plant |
| Na2HCO3 | Tianjin Yaohua Chemical Plant |
| Na2CO3 | Tianjin Yaohua Chemical Plant |
| Na2SO4 | Tianjin Yaohua Chemical Plant |
| CaCl2 | Harbin Xinda Chemical Plant |
| MgCl2 | Tianjin Fuchen Chemical Plant |
| Petroleum Ether | Shenyang W&S Chemical Plant |
| Purpose | Instrument Name | Types | Manufacturer |
|---|---|---|---|
| Preparation of emulsion | Digital display disperser | IKAT25 | IKA Company |
| Zeta potential | Micro electrophoresis apparatus | JS94H | Shanghai Zhongchen Digital technology equipment Co. Ltd. |
| Oil droplet size distribution | Laser particle size analyzer | BT-9300H | Dandong Better Science and Technology Co. Ltd. |
| Interfacial tension | Interface tension meter | XZD-5 | Beijing Hake Experimental Instrument Factory |
| Temperature control | Thermostatic water bath | S501-2 | Liaoyang Huaguang Instrument Factory |
| Micrographs observation | Biological microscope | IX73 | Shanghai Puhe Biotechnology Co. Ltd. |
| Weigh | Electronic balance | BS210S | Sartorius scientific Instruments Co. Ltd. |
| Quantitative transfer liquid | Micropipette | Eppendorf | Eppendorf China Co. Ltd. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Li, X.; Zhang, W.; Fu, C.; Wang, Y.; Fu, S. Study on Demulsification-Flocculation Mechanism of Oil-Water Emulsion in Produced Water from Alkali/Surfactant/Polymer Flooding. Polymers 2019, 11, 395. https://doi.org/10.3390/polym11030395
Huang B, Li X, Zhang W, Fu C, Wang Y, Fu S. Study on Demulsification-Flocculation Mechanism of Oil-Water Emulsion in Produced Water from Alkali/Surfactant/Polymer Flooding. Polymers. 2019; 11(3):395. https://doi.org/10.3390/polym11030395
Chicago/Turabian StyleHuang, Bin, Xiaohui Li, Wei Zhang, Cheng Fu, Ying Wang, and Siqiang Fu. 2019. "Study on Demulsification-Flocculation Mechanism of Oil-Water Emulsion in Produced Water from Alkali/Surfactant/Polymer Flooding" Polymers 11, no. 3: 395. https://doi.org/10.3390/polym11030395