Effect of Sodium Trimetaphosphate on Chitosan-Methylcellulose Composite Films: Physicochemical Properties and Food Packaging Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
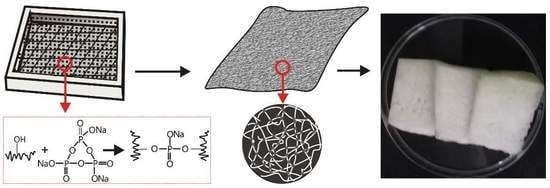
2.2. Fabrication of the Films
2.3. Response Surface Methodology
2.4. Characterization of Films
2.5. Antibacterial Activity
2.6. Enzymatic Degradation
2.7. Application on Fresh-Cut Wax Gourd
3. Results and Discussion
3.1. Response Surface Analysis
3.2. Characterization of Films
3.3. Antibacterial Activity
3.4. Enzymatic Degradation
3.5. Application on Fresh-Cut Wax Gourd
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crops Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Vitchayakitti, W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocolloids 2016, 61, 695–702. [Google Scholar] [CrossRef]
- Kisonen, V.; Prakobna, K.; Xu, C.; Salminen, A.; Mikkonen, K.S.; Valtakari, D.; Eklund, P.; Seppälä, J.; Tenkanen, M.; Willför, S. Composite films of nanofibrillated cellulose and O-acetyl galactoglucomannan (GGM) coated with succinic esters of GGM showing potential as barrier material in food packaging. J. Mater. Sci. 2015, 50, 3189–3199. [Google Scholar] [CrossRef]
- Ramziia, S.; Ma, H.; Yao, Y.; Wei, K.; Huang, Y. Enhanced antioxidant activity of fish gelatin–chitosan edible films incorporated with procyanidin. J. Appl. Polym. Sci. 2018, 135, 45781. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Zhang, A.; Li, K.; Oderinde, O.; Yao, F.; Fu, G. Zinc ion-induced formation of hierarchical N-succinyl chitosan film. J. Appl. Polym. Sci. 2017, 134, 44664. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food. Chem. 2017, 66, 395. [Google Scholar] [CrossRef]
- Owczarz, P.; Ziółkowski, P.; Dziubiński, M. The application of small-angle light scattering for rheo-optical characterization of chitosan colloidal solutions. Polymers 2018, 10, 431. [Google Scholar] [CrossRef]
- Paiva, D.; Gonçalves, C.; Vale, I.; Bastos, M.M.S.M.; Magalhães, F.D. Oxidized xanthan gum and chitosan as natural adhesives for cork. Polymers 2016, 8, 259. [Google Scholar] [CrossRef]
- Song, Z.; Li, G.; Guan, F.; Liu, W. Application of chitin/chitosan and their derivatives in the papermaking industry. Polymers 2018, 10, 389. [Google Scholar] [CrossRef]
- Qiao, C.; Ma, X.; Zhang, J.; Yao, J. Molecular interactions in gelatin/chitosan composite films. Food Chem. 2017, 235, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Anaya, P.; Cardenas, G.; Lavayen, V.; Garcia, A.; O’Dwyer, C. Chitosan gel film bandages: Correlating structure, composition, and antimicrobial properties. J. Appl. Polym. Sci. 2012, 128, 3939–3948. [Google Scholar] [CrossRef] [Green Version]
- Correia, C.O.; Caridade, S.G.; Mano, J.F. Chitosan membranes exhibiting shape memory capability by the action of controlled hydration. Polymers 2014, 6, 1178–1186. [Google Scholar] [CrossRef]
- Santos-López, G.; Argüelles-Monal, W.; Carvajal-Millan, E.; López-Franco, Y.L.; Recillas-Mota, M.T.; Lizardi-Mendoza, J. Aerogels from chitosan solutions in ionic liquids. Polymers 2017, 9, 722. [Google Scholar] [CrossRef]
- Nguyen, T.T.B.; Hein, S.; Ng, C.-H.; Stevens, W.F. Molecular stability of chitosan in acid solutions stored at various conditions. J. Appl. Polym. Sci. 2008, 107, 2588–2593. [Google Scholar] [CrossRef]
- Prasanna, K.; Sailaja, R.R.N. Blends of LDPE/chitosan using epoxy-functionalized LDPE as compatibilizer. J. Appl. Polym. Sci. 2012, 124, 3264–3275. [Google Scholar] [CrossRef]
- Khan, R.A.; Salmieri, S.; Dussault, D.; Sharmin, N.; Lacroix, M. Mechanical, barrier, and interfacial properties of biodegradable composite films made of methylcellulose and poly(caprolactone). J. Appl. Polym. Sci. 2012, 123, 1690–1697. [Google Scholar] [CrossRef]
- Bang, S.H.; Son, J.R.; Lee, S.Y.; Park, H.J. Preparation and characterization of composite gels and films containing gelatin and hydroxypropyl methylcellulose phthalate. J. Appl. Polym. Sci. 2014, 131, 569–582. [Google Scholar] [CrossRef]
- Sugantha Kumari, V.; Khaleel Basha, S.; Sudha, P.N. Physicochemical and morphological evaluation of chitosan/poly(vinyl alcohol)/methylcellulose chemically cross-linked ternary blends. Polym. Bull. 2011, 68, 1387–1393. [Google Scholar] [CrossRef]
- Abed, A.; Assoul, N.; Ba, M.; Derkaoui, S.M.; Portes, P.; Louedec, L.; Flaud, P.; Bataille, I.; Letourneur, D.; Meddahi-Pelle, A. Influence of polysaccharide composition on the biocompatibility of pullulan/dextran-based hydrogels. J. Biomed. Mater. Res. Part A 2011, 96, 535–542. [Google Scholar] [CrossRef]
- Ma, X.; Liu, C.; Anderson, D.P.; Chang, P.R. Porous cellulose spheres: Preparation, modification and adsorption properties. Chemosphere 2016, 165, 399–408. [Google Scholar] [CrossRef]
- Bejenariu, A.; Popa, M.; Dulong, V.; Picton, L.; Le Cerf, D. Trisodium trimetaphosphate crosslinked xanthan networks: Synthesis, swelling, loading and releasing behaviour. Polym. Bull. 2009, 62, 525–538. [Google Scholar] [CrossRef]
- Carbinatto, F.M.; de Castro, A.D.; Cury, B.S.; Magalhaes, A.; Evangelista, R.C. Physical properties of pectin-high amylose starch mixtures cross-linked with sodium trimetaphosphate. Int. J. Pharm. 2012, 423, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Gui-Jie, M.; Peng, W.; Xiang-Sheng, M.; Xing, Z.; Tong, Z. Crosslinking of corn starch with sodium trimetaphosphate in solid state by microwave irradiation. J. Appl. Polym. Sci. 2006, 102, 5854–5860. [Google Scholar] [CrossRef]
- Racksanti, A.; Janhom, S.; Punyanitya, S.; Watanesk, R.; Watanesk, S. An approach for preparing an absorbable porous film of silk fibroin-rice starch modified with trisodium trimetaphosphate. J. Appl. Polym. Sci. 2015. [Google Scholar] [CrossRef]
- Racksanti, A.; Janhom, S.; Punyanitya, S.; Watanesk, R.; Watanesk, S. Crosslinking density of silk fibroin-rice starch hydrogels modified with trisodium trimetaphosphate. Appl. Mech. Mater. 2013, 366–372. [Google Scholar] [CrossRef]
- Goncalves, I.; Nunes, C.; Mendes, S.; Martins, L.O.; Ferreira, P.; Coimbra, M.A. CotA laccase-ABTS/hydrogen peroxide system: An efficient approach to produce active and decolorized chitosan-genipin films. Carbohydr. Polym. 2017, 175, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yuan, L.; Lin, L. Novel chitosan film embedded with liposome-encapsulated phage for biocontrol of Escherichia coli O157:H7 in beef. Carbohydr. Polym. 2017, 177, 156–164. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Rodríguez, F.; Guarda, A.; Galotto, M.J. Antioxidant films based on cross-linked methyl cellulose and native Chilean berry for food packaging applications. Carbohydr. Polym. 2016, 136, 1052–1060. [Google Scholar] [CrossRef]
- Yu, S.-H.; Tsai, M.-L.; Lin, B.-X.; Lin, C.-W.; Mi, F.-L. Tea catechins-cross-linked methylcellulose active films for inhibition of light irradiation and lipid peroxidation induced β -carotene degradation. Food Hydrocolloids 2015, 44, 491–505. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W. Fabrication of antibacterial chitosan-PVA blended film using electrospray technique for food packaging applications. Int. J. Biol. Macromol. 2018, 107, 848–854. [Google Scholar] [CrossRef]
- Song, Z.; Li, F.; Guan, H.; Xu, Y.; Fu, Q.; Li, D. Combination of nisin and ε-polylysine with chitosan coating inhibits the white blush of fresh-cut carrots. Food Ctrl. 2017, 74, 34–44. [Google Scholar] [CrossRef]
- Aranaz, I.; Harris, R.; Navarro-García, F.; Heras, A.; Acosta, N. Chitosan based films as supports for dual antimicrobial release. Carbohydr. Polym. 2016, 146, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Akinosho, H.; Hawkins, S.; Wicker, L. Hydroxypropyl methylcellulose substituent analysis and rheological properties. Carbohydr. Polym. 2013, 98, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, S.; Chen, Y.; Zhang, L.; Kan, J.; Jin, C. Physical, mechanical and antioxidant properties of chitosan films grafted with different hydroxybenzoic acids. Food Hydrocolloids 2017, 71, 176–186. [Google Scholar] [CrossRef]
- Aziz, S.; Rasheed, M.; Ahmed, H. Synthesis of polymer nanocomposites based on [Methyl Cellulose](1−x):(CuS)x (0.02 M ≤ x ≤ 0.08 M) with desired optical band gaps. Polymers 2017, 9, 194. [Google Scholar] [CrossRef]
- Wei, X.-L.; Liang, S.; Xu, Y.-Y.; Sun, Y.-L.; An, J.-F.; Chao, Z.-S. Patching NaA zeolite membrane by adding methylcellulose into the synthesis gel. J. Membr. Sci. 2016, 530, 240–249. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Ge, H.; Chen, L.; Wang, J.; Zhang, S.; Guo, Y.; Li, Z.; Feng, X. Physical properties and antioxidant capacity of chitosan/epigallocatechin-3-gallate films reinforced with nano-bacterial cellulose. Carbohydr. Polym. 2017, 179, 207–220. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Wu, Q.; Gu, Y.; Kan, J.; Jin, C. Effect of protocatechuic acid incorporation on the physical, mechanical, structural and antioxidant properties of chitosan film. Food Hydrocolloids 2017, 73, 90–100. [Google Scholar] [CrossRef]
- Tunc, S.; Duman, O.; Polat, T.G. Effects of montmorillonite on properties of methyl cellulose/carvacrol based active antimicrobial nanocomposites. Carbohydr. Polym. 2016, 150, 259–268. [Google Scholar] [CrossRef]
- García, M.A.; Pinotti, A.; Martino, M.; Zaritzky, N. Electrically treated composite FILMS based on chitosan and methylcellulose blends. Food Hydrocolloids 2009, 23, 722–728. [Google Scholar]
- Pastor, C.; Sánchez-González, L.; Chiralt, A.; Cháfer, M.; González-Martínez, C. Physical and antioxidant properties of chitosan and methylcellulose-based films containing resveratrol. Food Hydrocolloids 2013, 30, 272–280. [Google Scholar] [CrossRef]
- Pinotti, A.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Study on microstructure and physical properties of composite films based on chitosan and methylcellulose. Food Hydrocolloids 2007, 21, 66–72. [Google Scholar] [CrossRef]








| Projects | Parameters |
|---|---|
| Addition of methylcellulose | 0, 30 wt %, 50 wt %, 70 wt %, and 100 wt % |
| STMP concentration | 0.1 wt %, 0.2 wt %, 0.3 wt %, 0.4 wt %, and 0.5 wt % |
| Crosslinking time | 0.5 h, 1.5 h, 2.5 h, 3.5 h, and 4.5 h |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 20.06 | 9 | 2.23 | 13.60 | 0.0012 |
| A-MC/(CS + MC) | 8.61 | 1 | 8.61 | 52.53 | 0.0002 |
| B-STMPC | 0.011 | 1 | 0.011 | 0.069 | 0.8009 |
| C-CT | 6.13 | 1 | 6.13 | 37.36 | 0.0005 |
| AB | 0.023 | 1 | 0.023 | 0.14 | 0.7220 |
| AC | 1.00 | 1 | 1.00 | 6.10 | 0.0428 |
| BC | 0.040 | 1 | 0.040 | 0.24 | 0.6364 |
| A2 | 3.32 | 1 | 3.32 | 20.23 | 0.0028 |
| B2 | 0.24 | 1 | 0.24 | 1.45 | 0.2678 |
| C2 | 0.41 | 1 | 0.41 | 2.51 | 0.1573 |
| Residual | 1.15 | 7 | 0.16 | ||
| Lack of Fit | 1.15 | 3 | 0.38 | ||
| Pure Error | 0.000 | 4 | 0.000 | ||
| Cor Total | 21.21 | 16 |
| Films | A500 | Color Parameters | |||
|---|---|---|---|---|---|
| L* | a* | b* | ∆E | ||
| CSF | 0.611 | 42.54 | 37.38 | 38.38 | − |
| CSMCF1 | 4.375 | 56.31 | 23 | 59.55 | 29.06 |
| CSMCF2 | 5.081 | 61.04 | 27.00 | 64.02 | 33.28 |
| CCSF | 5.472 | 79.81 | −0.84 | 25.32 | 54.96 |
| CCSMCF1 | 5.601 | 82.33 | −1.6 | 12.77 | 61.31 |
| CCSMCF2 | 5.632 | 86.21 | −2.00 | 12.41 | 64.28 |
| Samples | Solubility (%) | Inhibition Ratio (%) | Degradation Ratio (%) | |
|---|---|---|---|---|
| E. coli | S. aureus | |||
| CSF | 1.5 ± 0.03 | 99.8 | 99.9 | 2 ± 0.17 |
| CSMCF1 | 12 ± 0.11 | 99.1 | 99.6 | 3 ± 0.15 |
| CSMCF2 | 23 ± 0.07 | 99.2 | 99.2 | 6 ± 0.19 |
| CCSF | 1 ± 0.05 | 99.6 | 99.7 | 1.8 ± 0.08 |
| CCSMCF1 | 3 ± 0.09 | 99.1 | 99.0 | 1.7 ± 0.13 |
| CCSMCF2 | 6 ± 0.12 | 99.0 | 99.0 | 1.7 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liao, Y.; Wu, A.; Li, B.; Qian, J.; Ding, F. Effect of Sodium Trimetaphosphate on Chitosan-Methylcellulose Composite Films: Physicochemical Properties and Food Packaging Application. Polymers 2019, 11, 368. https://doi.org/10.3390/polym11020368
Wang H, Liao Y, Wu A, Li B, Qian J, Ding F. Effect of Sodium Trimetaphosphate on Chitosan-Methylcellulose Composite Films: Physicochemical Properties and Food Packaging Application. Polymers. 2019; 11(2):368. https://doi.org/10.3390/polym11020368
Chicago/Turabian StyleWang, Hongxia, Yu Liao, Ailiang Wu, Bing Li, Jun Qian, and Fuyuan Ding. 2019. "Effect of Sodium Trimetaphosphate on Chitosan-Methylcellulose Composite Films: Physicochemical Properties and Food Packaging Application" Polymers 11, no. 2: 368. https://doi.org/10.3390/polym11020368