Glass Fiber-Reinforced Phenol Formaldehyde Resin-Based Electrical Insulating Composites Fabricated by Selective Laser Sintering
Abstract
:1. Introduction
2. Experimental
2.1. Materials
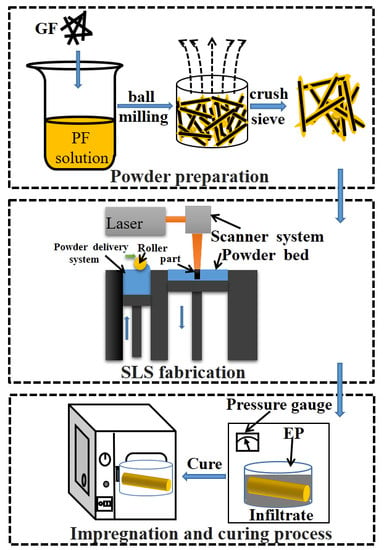
2.2. Experimental Process
2.3. Characterization
3. Results and Discussion
3.1. Powder Characteristics
3.2. Mechanical Properties
3.3. Electrical Properties
4. Conclusions
- The laser selective sintering fiber-reinforced resin composite material has a certain porosity and low strength. The post-treated impregnated epoxy resin can obtain a dense sample, and its bending strength and tensile strength were improved by 17.3% and 28%, respectively.
- For the GF/PF/EP three-phase composite materials containing 60 vol.%, 70 vol.%, and 80 vol.% of glass fibers, the tensile strength was 86.4, 92, and 96.2 MPa, respectively, and the flexural strength was 119, 129, and 137 MPa, respectively. Glass fiber is used as a reinforcing material, and as the GF content increases, the tensile strength and bending strength of the sample also increase.
- The electrical insulating properties of the composite material are enhanced with the increase of GF content. The breakdown voltage of the composite materials having a fiber content of 60 vol.%, 70 vol.%, and 80 vol.% was 29.6, 30.9, and 32.5 KV, and the DC volume resistivity was 19.978, 20.629 and 20.981 GΩ·m, respectively.
Author Contributions
Funding
Conflicts of Interest
References
- Abdalla, M.O.; Ludwick, A.; Mitchell, T. Boron-modified phenolic resins for high performance applications. Polymer 2003, 44, 7353–7359. [Google Scholar] [CrossRef]
- Martin, R. The Chemistry Of Phenolic Resins; Wiley: Hoboken, NJ, USA, 1956. [Google Scholar]
- Knop, A.; Scheib, W. Chemistry and Application of Phenolic Resins; Springer: Berlin/Heidelberg, Germany, 1979. [Google Scholar]
- Choi, M.H.; Jeon, B.H.; Chung, I.J. The effect of coupling agent on electrical and mechanical properties of carbon fiber/phenolic resin composites. Polymer 2000, 41, 3243–3252. [Google Scholar] [CrossRef]
- Simitzis, J.; Zoumpoulakis, L.; Soulis, S.; Triantou, D.; Pinaka, C. Electrical conductivity and mechanical strength of composites consisting of phenolic resin, carbon fibers, and metal particles. J. Appl. Polym. Sci. 2011, 121, 1890–1900. [Google Scholar] [CrossRef]
- Chen, Z.; Kang, G.; Liu, Y.; Wang, Y.; Wei, X.U. Experimental study on uniaxial time-dependent ratcheting of short glass fiber reinforced polyester resin matrix composites. Acta Mater. Compos. Sin. 2009, 26, 155–160. [Google Scholar]
- Joseph, S.; Sreekala, M.S.; Koshy, P.; Thomas, S. Mechanical properties and water sorption behavior of phenol–formaldehyde hybrid composites reinforced with banana fiber and glass fiber. J. Appl. Polym. Sci. 2010, 109, 1439–1446. [Google Scholar] [CrossRef]
- Cui, Y.; Chang, J.; Wang, W. Fabrication of Glass Fiber Reinforced Composites Based on Bio-Oil Phenol Formaldehyde Resin. Materials 2016, 9, 886. [Google Scholar] [CrossRef] [PubMed]
- Sreekala, M.; George, J.; Kumaran, M.; Thomas, S. The mechanical performance of hybrid phenol-formaldehyde-based composites reinforced with glass and oil palm fibres. Compos. Sci. Technol. 2002, 62, 339–353. [Google Scholar] [CrossRef]
- Rahim, T.N.A.T.; Abdullah, A.M.; Akil, H.M.; Mohamad, D.; Rajion, Z.A.; Rahim, T.N.A.T.; Abdullah, A.M.; Akil, H.M.; Mohamad, D.; Rajion, Z.A. The improvement of mechanical and thermal properties of polyamide 12 3D printed parts by fused deposition modelling. Express Polym. Lett. 2017, 11, 963–982. [Google Scholar] [CrossRef]
- Kumar, S. Selective laser sintering: A qualitative and objective approach. JOM 2003, 55, 43–47. [Google Scholar] [CrossRef]
- Bai, J.; Yuan, S.; Shen, F.; Zhang, B.; Chua, C.K.; Zhou, K.; Wei, J. Toughening of polyamide 11 with carbon nanotubes for additive manufacturing. Virtual Phys. Prototyp. 2017, 12, 235–240. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, Y.; Gao, C.; Feng, P.; Guo, W.; He, C.; Chen, J.; Shuai, C. Mechanism for corrosion protection of β-TCP reinforced ZK60 via laser rapid solidification. Int. J. Bioprint. 2018, 4, 4. [Google Scholar] [CrossRef]
- Ameen, W.; Ghaleb, A.M.; Alatefi, M.; Alkhalefah, H.; Alahmari, A. An overview of selective laser sintering and melting research using bibliometric indicators. Virtual Phys. Prototyp. 2018, 13, 282–291. [Google Scholar] [CrossRef]
- Goodridge, R.D.; Tuck, C.J.; Hague, R.J.M. Laser sintering of polyamides and other polymers. Prog. Mater Sci. 2012, 57, 229–267. [Google Scholar] [CrossRef]
- Yuan, S.; Shen, F.; Chua, C.K.; Zhou, K. Polymeric composites for powder-based additive manufacturing: Materials and applications. Prog. Polym. Sci. 2018, in press. [Google Scholar] [CrossRef]
- Qi, F.; Chen, N.; Wang, Q. Dielectric and piezoelectric properties in selective laser sintered polyamide11/BaTiO3/CNT ternary nanocomposites. Mater. Des. 2018, 143, 72–80. [Google Scholar] [CrossRef]
- Yuan, S.; Strobbe, D.; Kruth, J.P.; Puyvelde, P.V.; Bruggen, B.V.D. Production of polyamide-12 membranes for microfiltration through selective laser sintering. J. Membr. Sci. 2017, 525, 157–162. [Google Scholar] [CrossRef]
- Bai, J.; Hague, R.J.; Okamoto, M.; Goodridge, R.D.; Mo, S. Influence of carbon nanotubes on the rheology and dynamic mechanical properties of polyamide-12 for laser sintering. Polym. Test. 2014, 36, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Yan, C.; Shi, Y.; Wen, S.; Liu, J.; Shi, Y. Investigation into mechanical and microstructural properties of polypropylene manufactured by selective laser sintering in comparison with injection molding counterparts. Mater. Des. 2015, 82, 37–45. [Google Scholar] [CrossRef]
- Yan, M.; Tian, X.; Peng, G.; Li, D.; Zhang, X. High temperature rheological behavior and sintering kinetics of CF/PEEK composites during selective laser sintering. Compos. Sci. Technol. 2018, 165, 140–147. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Chen, J.; Huang, S. Experimental Investigation into the Selective Laser Sintering of High-Impact Polystyrene. J. Appl. Polym. Sci. 2010, 108, 535–540. [Google Scholar] [CrossRef]
- Laura, D.M.; Keskkula, H.; Barlow, J.W.; Paul, D.R. Effect of glass fiber surface chemistry on the mechanical properties of glass fiber reinforced, rubber-toughened nylon 6. Polymer 2002, 43, 4673–4687. [Google Scholar] [CrossRef]
- Wei, B.; Chang, Q.; Bao, C.; Dai, L.; Zhang, G.; Wu, F. Surface modification of filter medium particles with silane coupling agent KH550. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 276–280. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, J.; Zhang, B.; Wang, J.; Zhao, Y.; Liu, J. Preparation and Characterization of Silane Coupling Agent Modified Halloysite for Cr(VI) Removal. Ind. Eng. Chem. Res. 2011, 50, 10246–10252. [Google Scholar] [CrossRef]
- Chen, P.; Wu, H.; Zhu, W.; Yang, L.; Li, Z.; Yan, C.; Wen, S.; Shi, Y. Investigation into the processability, recyclability and crystalline structure of selective laser sintered Polyamide 6 in comparison with Polyamide 12. Polym. Test. 2018, 69, 366–374. [Google Scholar] [CrossRef]
- Manshoori Yeganeh, A.; Movahhedy, M.; Khodaygan, S. An efficient scanning algorithm for improving accuracy based on minimising part warping in selected laser sintering process. Virtual Phys. Prototyp. 2018, 14, 59–78. [Google Scholar] [CrossRef]
- Zou, L.; Bloebaum, R.; Bachus, K. Reproducibility of techniques using Archimedes’ principle in measuring cancellous bone volume. Med. Eng. Phys. 1997, 19, 63–68. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; Wang, J.; Guo, Q.; Li, J. Size-controlled synthesis of dispersed equiaxed amorphous TiO2 nanoparticles. Ceram. Int. 2015, 41, 9057–9062. [Google Scholar] [CrossRef]
- Shang, R.; Hou, J.; Wang, L.; Yuan, J.; Wang, X. Surface Modification of Potassium Ionic Sieve by Silane Coupling Agent KH-550. Mater. Rev. 2011, 2, 25–27. [Google Scholar]
- Varga, C.; Miskolczi, N.; Bartha, L.; Lipóczi, G. Improving the mechanical properties of glass-fibre-reinforced polyester composites by modification of fibre surface. Mater. Des. 2010, 31, 185–193. [Google Scholar] [CrossRef]
- Kim, S.Y.; Woo, S.K.; Han, I.S. Novel Phenol Resin Carbonizing Method for Carbon Interlayer Coating between Reinforcing Fiber and Matrix in Fiber Reinforced Ceramic Composite. J. Korean Ceram. Soc. 2009, 46, 301–305. [Google Scholar] [CrossRef]
- Jansson, A.; Pejryd, L. Characterisation of carbon fibre-reinforced polyamide manufactured by selective laser sintering. Addit. Manuf. 2016, 9, 7–13. [Google Scholar] [CrossRef]
- Hooreweder, B.V.; Kruth, J.P. High cycle fatigue properties of selective laser sintered parts in polyamide 12. CIRP Ann. Manuf. Technol. 2014, 63, 241–244. [Google Scholar] [CrossRef] [Green Version]
- Joseph, S.; Sreekala, M.S.; Oommen, Z.; Koshy, P.; Thomas, S. A comparison of the mechanical properties of phenol formaldehyde composites reinforced with banana fibres and glass fibres. Compos. Sci. Technol. 2002, 62, 1857–1868. [Google Scholar] [CrossRef]
- Marshall, D.B.; Cox, B.N.; Evans, A.G. The mechanics of matrix cracking in brittle-matrix fiber composites. Acta Metall. 1985, 33, 2013–2021. [Google Scholar] [CrossRef]
- Kumar, N.M.; Reddy, G.V.; Naidu, S.V.; Rani, T.S.; Subha, M.C.S. Mechanical Properties of Coir/Glass Fiber Phenolic Resin Based Composites. J. Reinf. Plast. Compos. 2009, 28, 2605–2613. [Google Scholar] [CrossRef]
- Naito, K.; Kagawa, Y.; Utsuno, S.; Naganuma, T.; Kurihara, K. Dielectric properties of woven fabric glass fiber reinforced polymer-matrix composites in the THz frequency range. Compos. Sci. Technol. 2009, 69, 2027–2029. [Google Scholar] [CrossRef]
- Brosseau, C.; Beroual, A. Computational electromagnetics and the rational design of new dielectric heterostructures. Prog. Mater Sci. 2003, 48, 373–456. [Google Scholar] [CrossRef]
- Fuoss, R.M. Dielectric Materials and Applications; MIT Press: Cambridge, MA, USA, 1954; p. 1714. [Google Scholar]
- Shtaif, M.; Mecozzi, A. Study of the frequency autocorrelation of the differential group delay in fibers with polarization mode dispersion. Opt. Lett. 2000, 25, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.; Wool, R.P.; Xiao, J.Q. Electrical properties of chicken feather fiber reinforced epoxy composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 229–233. [Google Scholar] [CrossRef]









| Properties | Parameters |
|---|---|
| The average particle size (μm) | 22.1 |
| Softening temperature (°C) | 98~115 |
| Urotropine (%) | 8~9 |
| Density (g/cm3) | 1.22 |
| Material appearance | Light yellow to brown transparent solid |
| Laser Power (W) | Scan Velocity (mm/s) | Scan Spacing (mm) | Layer Thickness (mm) | Sintering Result |
|---|---|---|---|---|
| 10 | 3000 | 0.2 | 0.1 | Fragile |
| 12 | 2500 | 0.3 | 0.1 | Fragile |
| 12 | 3000 | 0.2 | 0.1 | Serious warped |
| 12 | 3500 | 0.1 | 0.1 | Serious warped |
| 14 | 2500 | 0.1 | 0.1 | Slightly warped |
| 14 | 3000 | 0.2 | 0.1 | Well formed |
| 14 | 3500 | 0.3 | 0.1 | Well formed |
| 16 | 3000 | 0.2 | 0.1 | Low precision |
| GF Content/vol.% | 60 | 70 | 80 |
|---|---|---|---|
| Porosity/% | 53.5 | 54.9 | 58.7 |
| GF Content/vol.% | Tensile Strength/MPa | Bending Strength/MPa |
|---|---|---|
| 60 | 86.4 | 119 |
| 70 | 92 | 129 |
| 80 | 96.2 | 137 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhou, W.; Yang, L.; Chen, P.; Yan, C.; Cai, C.; Li, H.; Li, L.; Shi, Y. Glass Fiber-Reinforced Phenol Formaldehyde Resin-Based Electrical Insulating Composites Fabricated by Selective Laser Sintering. Polymers 2019, 11, 135. https://doi.org/10.3390/polym11010135
Li Z, Zhou W, Yang L, Chen P, Yan C, Cai C, Li H, Li L, Shi Y. Glass Fiber-Reinforced Phenol Formaldehyde Resin-Based Electrical Insulating Composites Fabricated by Selective Laser Sintering. Polymers. 2019; 11(1):135. https://doi.org/10.3390/polym11010135
Chicago/Turabian StyleLi, Zhaoqing, Wangbing Zhou, Lei Yang, Peng Chen, Chunze Yan, Chao Cai, Hua Li, Lee Li, and Yusheng Shi. 2019. "Glass Fiber-Reinforced Phenol Formaldehyde Resin-Based Electrical Insulating Composites Fabricated by Selective Laser Sintering" Polymers 11, no. 1: 135. https://doi.org/10.3390/polym11010135