Novel Intumescent Flame Retardant Masterbatch Prepared through Different Processes and Its Application in EPDM/PP Thermoplastic Elastomer: Thermal Stability, Flame Retardancy, and Mechanical Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Flame Retardant Masterbatches
2.2.1. Fabrication of Reactive Extruded Flame Retardant Masterbatches
2.2.2. Fabrication of Compound Flame Retardant Masterbatches
2.3. Preparation of the Flame Retardant Composites
2.4. Measurements and Characterization
2.4.1. Fourier transform infrared spectroscopy (FTIR)
2.4.2. Calculations of Conversion Rate
2.4.3. Scanning Electronic Microscopy (SEM)
2.4.4. Thermogravimetric Analysis (TGA)
2.4.5. Limiting Oxygen Index (LOI)
2.4.6. UL-94 Vertical Burning Test
2.4.7. Cone Calorimeter Test (CCT)
2.4.8. Mechanical Properties
3. Results and Discussion
3.1. FTIR Analysis
3.2. Thermal Stability
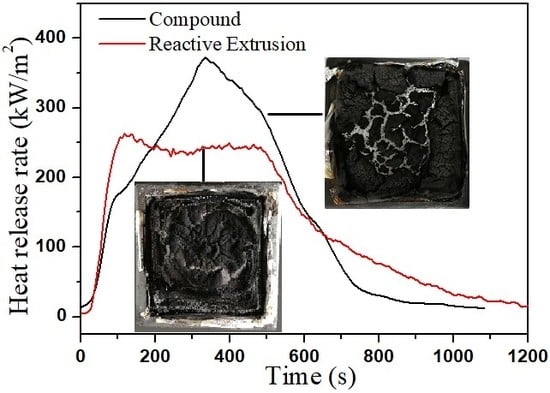
3.3. Flame Retardancy
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Legge, N.R. Thermoplastic elastomers-three decades of progress. Rubber Chem. Technol. 1989, 62, 529–547. [Google Scholar] [CrossRef]
- Amin, S.; Amin, M. Thermoplastic elastomeric (TPE) materials and their use in outdoor electrical insulation. Rev. Adv. Mater. 2011, 29, 15–30. [Google Scholar]
- Naskar, A.K.; Bhowmick, A.K.; De, S.K. Thermoplastic elastomeric composition based on ground rubber tire. Polym. Eng. Sci. 2001, 41, 1087–1098. [Google Scholar] [CrossRef]
- Kresge, E.N. Polyolefin thermoplastic elastomer blends. Rubber Chem. Technol. 1991, 64, 469–480. [Google Scholar] [CrossRef]
- Lima, P.; da Silva, S.P.M.; Oliveira, J.; Costa, V. Rheological properties of ground tyre rubber based thermoplastic elastomeric blends. Polym. Test. 2015, 45, 58–67. [Google Scholar] [CrossRef]
- Romin, R.; Nakason, C.; Thitithammawong, A. Thermoplastic elastomer based on epoxidized natural rubber/polyamide-12 and co-polyamide-12 blends. Adv. Mater. Res. 2012, 626, 58–61. [Google Scholar] [CrossRef]
- Bazgir, S.; Katbab, A.-A.; Nazockdast, H.J. Silica reinforced dynamically vulcanized EPDM/PP thermoplastic elastomers: Morphology, rheology and dynamic mechanical properties. J. Appl. Polym. Sci. 2004, 92, 2000–2007. [Google Scholar] [CrossRef]
- Litvinov, V. EPDM/PP thermoplastic vulcanizates as studied by proton NMR relaxation: Phase composition, molecular mobility, network structure in the rubbery phase, and network heterogeneity. Macromolecules 2006, 39, 8727–8741. [Google Scholar] [CrossRef]
- Goharpey, F.; Mirzadeh, A.; Sheikh, A.; Katbab, A.-A. Study on microstructure, rheological, and mechanical properties of cellulose short fiber reinforced TPVs based on EPDM/PP. Polym. Compos. 2010, 30, 182–187. [Google Scholar] [CrossRef]
- Katbab, A.-A.; Nazockdast, H.; Bazgir, S. Carbon black-reinforced dynamically cured EPDM/PP thermoplastic elastomers. I. Morphology, rheology, and dynamic mechanical properties. J. Appl. Polym. Sci. 2015, 75, 1127–1137. [Google Scholar] [CrossRef]
- Antunes, C.F.; Machado, A.V.; Van Duin, M. Morphology development and phase inversion during dynamic vulcanisation of EPDM/PP blends. Eur. Polym. J. 2011, 47, 1447–1459. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, B.; Zhou, N.; Yu, F.; Zhang, H. Flame-retardant olefin block copolymer composites with novel halogen-free intumescent flame retardants based on the composition of melamine phosphate and pentaerythritol. J. Appl. Polym. Sci. 2014, 131, 40066. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, C.; Chen, X. Effects of carbon fibers on the flammability and smoke emission characteristics of halogen-free thermoplastic polyurethane/ammonium polyphosphate. J. Mater. Sci. 2016, 51, 3762–3771. [Google Scholar] [CrossRef]
- Levchik, S.V.; Levchik, G.F.; Balabanovich, A.I.; Camino, G.; Costa, L. Mechanistic study of combustion performance and thermal decomposition behaviour of nylon 6 with added halogen-free fire retardants. Polym. Degrad. Stab. 1996, 54, 217–222. [Google Scholar] [CrossRef]
- Zhang, Q.; Tian, M.; Wu, Y.; Zhang, L. Effect of particle size on the properties of Mg(OH)2-filled rubber composites. J. Appl. Polym. Sci. 2010, 94, 2341–2346. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Adelnia, H.; Mohamad Sadeghi Gity, M.; Zafari, F. Intumescent flame retardant polyurethane/starch composites: Thermal, mechanical, and rheological properties. J. Appl. Polym. Sci. 2014, 131, 243–254. [Google Scholar] [CrossRef]
- He, W.; Qi, F.; Wang, N.; Cheng, X.; Zhang, K.; Guo, J. The influence of thermal oxidative ageing on flame retardancy, thermal and mechanical properties of LGFPP/IFR composites. J. Therm. Anal. Calorim. 2017, 131, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Feng, N.; Chang, S.; Zhang, G.; Li, H.; Lv, H. Intumescent flame retardant TPO composites: Flame retardant properties and morphology of the charred layer. J. Appl. Polym. Sci. 2012, 124, 2071–2079. [Google Scholar] [CrossRef]
- Enescu, D.; Frache, A.; Lavaselli, M.; Monticelli, O.; Marino, F. Novel phosphorous–nitrogen intumescent flame retardant system. Its effects on flame retardancy and thermal properties of polypropylene. Polym. Degrad. Stab. 2013, 98, 297–305. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Liu, Y.; Yin, H.; Aelmens, N.; Kierkels, R. Performance of an intumescent-flameretardant master batch synthesized by twin-screw reactive extrusion: Effect of the polypropylene carrier resin. Polym. Int. 2004, 53, 439–448. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Wang, Q.; Yin, H.; Aelmens, N.; Kierkels, R. Performance of intumescent flame retardant master batch synthesized through twin-screw reactively extruding technology: Effect of component ratio. Polym. Degrad. Stab. 2003, 81, 215–224. [Google Scholar] [CrossRef]
- Hamzah, M.; Jaafar, M. Properties of flame-retardant fillers in polypropylene/ethylene propylene diene monomer composites. J. Thermoplast. Compos. Mater. 2013, 26, 1223–1236. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, L.; Lin, L.; Wang, Y. Synergistic effect of layered nanofillers in intumescent flame-retardant EPDM: Montmorillonite versus layered double hydroxides. Ind. Eng. Chem. Res. 2013, 52, 8454–8463. [Google Scholar] [CrossRef]
- Shimpi, N.G.; Mali, A.D.; Sonawane, H.A.; Mishra, S. Effect of nBaCO3 on mechanical, thermal and morphological properties of isotactic PP-EPDM blend. Polym. Bull. 2014, 71, 2067–2080. [Google Scholar] [CrossRef]
- Nguyen, C.; Kim, J. Thermal stabilities and flame retardancies of nitrogen–phosphorus flame retardants based on bisphosphoramidates. Polym. Degrad. Stab. 2008, 93, 1037–1043. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, P.; Hu, Y.; Hu, K. Thermal degradation study of intumescent flame retardants by TG and FTIR: Melamine phosphate and its mixture with pentaerythritol. J. Anal. Appl. Pyrolysis 2009, 81, 207–214. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, M.; Liu, N.; Dang, P.; Xu, Y.; Chen, X.; Wang, Z.; He, J. Combustion characteristics and thermal properties of HDPE/EVA blends containing magnesium hydroxide. J. Thermoplast. Compos. Mater. 2016, 24, 1–21. [Google Scholar]
- Zhang, J.; Wang, X.; Zhang, F.; Horrocks, A.R. Estimation of heat release rate for polymer-filler composites by cone calorimetry. Polym. Test. 2004, 23, 225–230. [Google Scholar] [CrossRef]
- Tang, M.; Qi, F.; Chen, M.; Sun, Z.; Xu, Y.; Chen, X.; Zhang, Z.; Shen, R. Synergistic effects of ammonium polyphosphate and red phosphorus with expandable graphite on HDPE/EVA blends. Polym. Adv. Technol. 2016, 27, 52–60. [Google Scholar] [CrossRef]
- Zanetti, M.; Bracco, P.; Costa, L. Thermal degradation behaviour of PE/clay nanocomposites. Polym. Degrad. Stab. 2004, 85, 657–665. [Google Scholar] [CrossRef]
- Chen, X.; Yu, J.; Guo, S. Thermal oxidative degradation kinetics of PP and PP/Mg(OH)2 flame retardant composites. J. Appl. Polym. Sci. 2007, 103, 1978–1984. [Google Scholar] [CrossRef]
- Jiang, J.; Cheng, Y.; Liu, Y.; Wang, Q.; He, Y.; Wang, B. Intergrowth charring for flame-retardant glass fabric-reinforced epoxy resin composites. J. Mater. Chem. A 2015, 3, 4284–4290. [Google Scholar] [CrossRef]













| MP/PER Ratio | T5% (°C) | Tmax (°C) | Residues at 600 °C (%) |
|---|---|---|---|
| 1.0 | 320.4 ± 1.1 | 493.4 ± 1.3 | 12.7 ± 0.3 |
| 1.2 | 324.6 ± 0.9 | 491.3 ± 1.5 | 16.9 ± 0.2 |
| 1.4 | 344.3 ± 0.8 | 496.6 ± 0.7 | 15.4 ± 0.5 |
| 1.6 | 347.0 ± 1.0 | 495.8 ± 1.2 | 18.2 ± 0.3 |
| 1.8 | 350.9 ± 0.3 | 500.8 ± 1.6 | 17.6 ± 0.8 |
| 2.0 | 334.0 ± 1.8 | 464.3 ± 2.2 | 11.6 ± 1.5 |
| IFR Content (wt %) | T5% (°C) | Tmax (°C) | Residues at 600 °C (%) |
|---|---|---|---|
| 0 | 273.0 ± 1.3 | 493.5 ± 0.8 | 0.6 ± 0.05 |
| 20 | 315.8 ± 2.2 | 493.9 ± 0.4 | 7.8 ± 0.11 |
| 25 | 323.7 ± 2.0 | 493.8 ± 0.7 | 8.1 ± 0.07 |
| 30 | 350.6 ± 1.5 | 497.2 ± 1.1 | 12.1 ± 0.21 |
| 35 | 348.4 ± 1.7 | 497.8 ± 0.6 | 13.4 ± 0.31 |
| IFR Content (wt %) | Reactive EPDM/PP/IFR | Compound EPDM/PP/IFR | ||
|---|---|---|---|---|
| LOI (%) | UL-94 | LOI (%) | UL-94 | |
| 0 | 18.5 ± 0.1 | failed | 18.5 ± 0.1 | failed |
| 20 | 22.4 ± 0.1 | failed | 20.3 ± 0.1 | failed |
| 25 | 25.5 ± 0.1 | failed | 22.7 ± 0.1 | failed |
| 30 | 28.2 ± 0.1 | V-1 | 24.5 ± 0.1 | failed |
| 35 | 29.7 ± 0.1 | V-0 | 25.8 ± 0.1 | failed |
| Samples | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | pSPR (m2/s) |
|---|---|---|---|---|
| Compound | 16 ± 2 | 16 ± 2 | 372.1 ± 11.3 | 164.5 ± 9.8 |
| Reactive | 20 ± 3 | 20 ± 5 | 262.8 ± 11.3 | 144.2 ± 4.3 |
| IFR Contents (wt %) | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | pSPR (m2/s) |
|---|---|---|---|---|
| 0 | 11 ± 2 | 502.3 ± 13.3 | 145.4 ± 10.1 | 0.0435 ± 0.002 |
| 20 | 18 ± 1 | 281.2 ± 10.4 | 170.6 ± 6.6 | 0.0415 ± 0.004 |
| 25 | 20 ± 3 | 262.8 ± 11.3 | 144.2 ± 4.3 | 0.0330 ± 0.002 |
| 30 | 27 ± 2 | 194.8 ± 10.2 | 128.7 ± 5.3 | 0.0229 ± 0.001 |
| 35 | 32 ± 3 | 163.2 ± 9.7 | 102.7 ± 2.7 | 0.0181 ± 0.003 |
| Samples | Tensile Strength (MPa) | Bending Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| Reactive | 24.8 ± 0.6 | 15.0 ± 1.2 | 483.2 ± 8.3 |
| Compound | 22.5 ± 0.8 | 17.6 ± 1.8 | 328.5 ± 12.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Zhou, Y.; Chen, X.; Guo, J.; Zhou, D.; Chen, S.; Wang, M.; Li, L. Novel Intumescent Flame Retardant Masterbatch Prepared through Different Processes and Its Application in EPDM/PP Thermoplastic Elastomer: Thermal Stability, Flame Retardancy, and Mechanical Properties. Polymers 2019, 11, 50. https://doi.org/10.3390/polym11010050
He W, Zhou Y, Chen X, Guo J, Zhou D, Chen S, Wang M, Li L. Novel Intumescent Flame Retardant Masterbatch Prepared through Different Processes and Its Application in EPDM/PP Thermoplastic Elastomer: Thermal Stability, Flame Retardancy, and Mechanical Properties. Polymers. 2019; 11(1):50. https://doi.org/10.3390/polym11010050
Chicago/Turabian StyleHe, Weidi, Ying Zhou, Xiaolang Chen, Jianbing Guo, Dengfeng Zhou, Shaopeng Chen, Meng Wang, and Lingtong Li. 2019. "Novel Intumescent Flame Retardant Masterbatch Prepared through Different Processes and Its Application in EPDM/PP Thermoplastic Elastomer: Thermal Stability, Flame Retardancy, and Mechanical Properties" Polymers 11, no. 1: 50. https://doi.org/10.3390/polym11010050