Hierarchical Porous Polyamide 6 by Solution Foaming: Synthesis, Characterization and Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
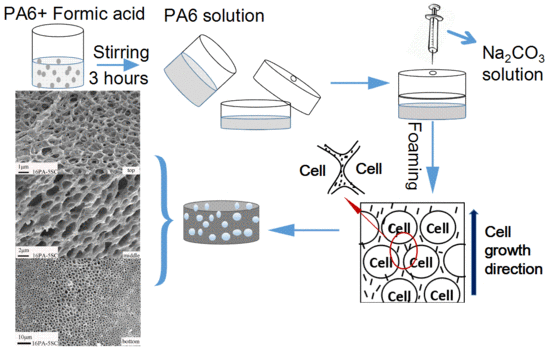
2.2. Preparation of PA Foams
2.3. Characterizations
3. Results and Discussion
3.1. Morphologies
3.2. DSC Analysis
3.3. WAXD Analysis
3.4. Thermal Conductivity
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wypych, G. Polyamide 6. In Handbook of Polymers; Wypych, G., Ed.; Elsevier: Oxford, UK, 2012; pp. 209–214. [Google Scholar]
- Schüler, F.; Schamel, D.; Salonen, A.; Drenckhan, W.; Gilchrist, M.D.; Stubenrauch, C. Synthesis of macroporous polystyrene by the polymerization of foamed emulsions. Angew. Chem. Int. Ed. 2012, 51, 2213–2217. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.J. Polymer Foams Handbook; Elsevier: Amsterdam, The Netherlands, 2007; p. 85. [Google Scholar]
- Colton, J.S.; Suh, N.P. Nucleation of microcellular foam: Theory and practice. Polym. Eng. Sci. 2010, 27, 500–503. [Google Scholar] [CrossRef]
- Liu, S.; Duvigneau, J.; Vancso, G.J. Nanocellular polymer foams as promising high performance thermal insulation materials. Eur. Polym. J. 2015, 65, 33–45. [Google Scholar] [CrossRef]
- Moglia, R.; Whitely, M.; Dhavalikar, P.; Robinson, J.; Pearce, H.; Brooks, M.; Stuebben, M.; Cordner, N.; Cosgriffhernandez, E. Injectable polymerized high internal phase emulsions with rapid in situ curing. Biomacromolecules 2014, 15, 2870–2878. [Google Scholar] [CrossRef] [PubMed]
- Lumelsky, Y.; Zoldan, J.; Levenberg, S.; Silverstein, M.S. Porous polycaprolactone-polystyrene semi-interpenetrating polymer networks synthesized within high internal phase emulsions. Macromolecules 2008, 41, 1469–1474. [Google Scholar] [CrossRef]
- Tai, H.; Sergienko, A.; Silverstein, M.S. Organic-inorganic networks in foams from high internal phase emulsion polymerizations. Polymer 2001, 42, 4473–4482. [Google Scholar] [CrossRef]
- Rahman, M.A.; Renna, L.A.; Venkataraman, D.; Desbois, P.; Lesser, A.J. High crystalline, porous polyamide 6 by anionic polymerization. Polymer 2018, 138, 8–16. [Google Scholar] [CrossRef]
- Wang, L.; Schiraldi, D.A.; Sanchez-Soto, M. Foam-like xanthan gum/clay aerogel composites and tailoring properties by blending with agar. Ind. Eng. Chem. Res. 2014, 53, 7680–7687. [Google Scholar] [CrossRef]
- Wang, L.; Sánchez-Soto, M.; Maspoch, M.L. Polymer/clay aerogel composites with flame retardant agents: Mechanical, thermal and fire behavior. Mater. Des. 2013, 52, 609–614. [Google Scholar] [CrossRef]
- Rudaz, C.; Courson, R.; Bonnet, L.; Calasetienne, S.; Sallée, H.; Budtova, T. Aeropectin: Fully biomass-based mechanically strong and thermal superinsulating aerogel. Biomacromolecules 2014, 15, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Grishechko, L.I.; Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Kuznetsov, B.N.; Pizzi, A.; Celzard, A. New tannin-lignin aerogels. Ind. Crop. Prod. 2013, 41, 347–355. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, J.; Park, D.; Han, H. A novel synthesis method for an open-cell microsponge polyimide for heat insulation. Polymer 2015, 56, 68–72. [Google Scholar] [CrossRef]
- Verdolotti, L.; Lavorgna, M.; Lamanna, R.; Di Maio, E.; Iannace, S. Polyurethane-silica hybrid foam by sol-gel approach: Chemical and functional properties. Polymer 2015, 56, 20–28. [Google Scholar] [CrossRef]
- Rafique, F.Z.; Vasanthan, N. Crystallization, crystal structure, and isothermal melt crystallization kinetics of novel polyamide 6/SiO2 nanocomposites prepared using the sol-gel technique. J. Phys. Chem. B 2014, 118, 9486–9495. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.J.; Park, J.H.; Sung, Y.T.; Hwang, D.H.; Kim, W.N.; Lee, H.S. Properties of water-blown rigid polyurethane foams with reactivity of raw materials. J. Appl. Polym. Sci. 2010, 93, 2334–2342. [Google Scholar] [CrossRef]
- Realinho, V.; Haurie, L.; Antunes, M.; Velasco, J.I. Thermal stability and fire behaviour of flame retardant high density rigid foams based on hydromagnesite-filled polypropylene composites. Compos. Part B 2014, 58, 553–558. [Google Scholar] [CrossRef]
- Zhang, D.; Burkes, W.L.; Schoener, C.A.; Grunlan, M.A. Porous inorganic-organic shape memory polymers. Polymer 2012, 53, 2935–2941. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Ikeya, M.; Okamoto, M. Foam processing and cellular structure of polylactide-based nanocomposites. Polymer 2006, 47, 5350–5359. [Google Scholar]
- Bian, J.; Ye, S.R.; Feng, L.X. Heterogeneous nucleation on the crystallization poly(ethylene terephthalate). J. Polym. Sci. Part B Polym. Phys. 2003, 41, 2135–2144. [Google Scholar] [CrossRef]
- Abbasi, H.; Antunes, M.; Velasco, J.I. Effects of carbon nanotubes/graphene nanoplatelets hybrid systems on the structure and properties of polyetherimide-based foams. Polymers 2018, 10, 348. [Google Scholar] [CrossRef]
- Huang, Z.; Yin, Q.; Wang, Q.; Wang, P.; Liu, T.; Qian, L. Mechanical properties and crystallization behavior of three kinds of straws/nylon 6 composites. Int. J. Boil. Macromol. 2017, 103, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Delkash, M.; Naderi, G.; Sahraieyan, R.; Esmizadeh, E. Crystallization, structural and mechanical properties of PA6/PC/NBR ternary blends: Effect of NBR-g-GMA compatibilizer and organoclay. Sci. Eng. Compos. Mater. 2017, 24, 669–678. [Google Scholar] [CrossRef]
- Diao, J.; Salazar, R.; Kelton, K.F.; Gelb, L.D. Impact of diffusion on concentration profiles around near-critical nuclei and implications for theories of nucleation and growth. Acta Mater. 2008, 56, 2585–2591. [Google Scholar] [CrossRef]
- Shan, G.F.; Yang, W.; Tang, X.G.; Yang, M.B.; Xie, B.H.; Fu, Q.; Mai, Y.W. Multiple melting behaviour of annealed crystalline polymers. Polym. Test. 2010, 29, 273–280. [Google Scholar] [CrossRef]
- Antonio, R.R.J.; Cristina, S.A.; Michel, D.; Rodríguez-Perez, M.A.; Leo, G. Production, cellular structure and thermal conductivity of microcellular (methyl methacrylate)-(butyl acrylate)-(methyl methacrylate) triblock copolymers. Polym. Int. 2011, 60, 146–152. [Google Scholar]
- Notario, B.; Pinto, J.; Solorzano, E.; Saja, J.A.D.; Dumon, M.; Rodríguez-Pérez, M.A. Experimental validation of the knudsen effect in nanocellular polymeric foams. Polymer 2015, 56, 57–67. [Google Scholar] [CrossRef]
- Solórzano, E.; Reglero, J.A.; Rodríguez-Pérez, M.A.; Lehmhus, D.; Wichmann, M.; Saja, J.A.D. An experimental study on the thermal conductivity of aluminium foams by using the transient plane source method. Int. J. Heat Mass Transf. 2008, 51, 6259–6267. [Google Scholar] [CrossRef]
- Luo, Y.; Ye, C. Using nanocapsules as building blocks to fabricate organic polymer nanofoam with ultra low thermal conductivity and high mechanical strength. Polymer 2012, 53, 5699–5705. [Google Scholar] [CrossRef]
- Qiu, L.; Zheng, X.H.; Zhu, J.; Tang, D.W.; Yang, S.Y.; Hu, A.J.; Wang, L.L.; Li, S.S. Thermal transport in high-strength polymethacrylimide (pmi) foam insulations. Int. J. Thermophys. 2015, 36, 2523–2534. [Google Scholar] [CrossRef]
- Saruhan, B.; Ryukhtin, V.; Kelm, K. Correlation of thermal conductivity changes with anisotropic nano-pores of eb-pvd deposited fysz-coatings. Surf. Coat. Technol. 2011, 205, 5369–5378. [Google Scholar] [CrossRef]
- Sun, Y.; Amirrasouli, B.; Razavi, S.B.; Li, Q.M.; Lowe, T.; Withers, P.J. The variation in elastic modulus throughout the compression of foam materials. Acta Mater. 2016, 110, 161–174. [Google Scholar] [CrossRef]
- Sotomayor, O.E.; Tippur, H.V. Role of cell regularity and relative density on elasto-plastic compression response of random honeycombs generated using voronoi diagrams. Int. J. Solids Struct. 2014, 51, 3776–3786. [Google Scholar] [CrossRef]
- Root, S.; Haill, T.A.; Lane, J.M.D.; Thompson, A.P.; Grest, G.S.; Schroen, D.G.; Mattsson, T.R. Shock compression of hydrocarbon foam to 200 gpa: Experiments, atomistic simulations, and mesoscale hydrodynamic modeling. J. Appl. Phys. 2013, 114, 103502. [Google Scholar] [CrossRef]
- Jaeger, P.D.; Huisseune, H.; Ameel, B.; Paepe, M.D. An experimentally validated and parameterized periodic unit-cell reconstruction of open-cell foams. J. Appl. Phys. 2011, 109, 805–806. [Google Scholar] [CrossRef]







| Samples | X1 (%) | X2 (%) | X (%) | Tm1 (°C) | Tm2 (°C) | Tc1 (°C) | Tc2 (°C) |
|---|---|---|---|---|---|---|---|
| Raw PA6 | 25.4 | / | 25.4 | 226.3 | / | 165.5 | / |
| 16PA-3SC | 13.6 | 34.7 | 48.3 | 215.9 | 263.9 | 178.2 | 237.9 |
| 16PA-5SC | 20.9 | 29.8 | 50.7 | 215.5 | 264.3 | 176.1 | 233.4 |
| 16PA-7SC | 17.1 | 40.2 | 57.3 | 215.8 | 264.0 | 178.5 | 234.3 |
| 16PA-9SC | 17.3 | 31.6 | 48.9 | 217.8 | 265.3 | 174.0 | / |
| 12PA-5SC | 24.1 | 29.0 | 53.1 | 216.9 | 265.6 | 176.4 | 227.9 |
| 14PA-5SC | 9.4 | 45.4 | 54.8 | 216.7 | 264.1 | 179.5 | 233.6 |
| 18PA-5SC | 26.7 | 35.2 | 61.9 | 216.8 | 264.0 | 177.8 | 240.4 |
| Samples | ρb (g/cm3) | E (MPa) | σ60% (MPa) | Ea (kJ) |
|---|---|---|---|---|
| 16PA-3SC | 0.096 ± 0.002 | 1.38 ± 0.15 | 1.08 ± 0.05 | 231.8 ± <0.1 |
| 16PA-5SC | 0.095 ± 0.002 | 1.33 ± 0.10 | 1.23 ± 0.06 | 239.2 ± <0.1 |
| 16PA-7SC | 0.092 ± 0.002 | 1.29 ± 0.07 | 1.12 ± 0.03 | 238.8 ± <0.1 |
| 16PA-9SC | 0.090 ± 0.001 | 1.05 ± 0.06 | 0.96 ± 0.02 | 198.2 ± <0.1 |
| 12PA-5SC | 0.066 ±< 0.001 | 1.02 ± 0.07 | 0.92 ± 0.02 | 199.5 ± <0.1 |
| 14PA-5SC | 0.082 ± 0.001 | 1.14 ± 0.04 | 1.21 ± 0.04 | 237.1 ± <0.1 |
| 18PA-5SC | 0.101 ±<0.001 | 4.74 ± 0.08 | 1.53 ± 0.05 | 403.7 ± <0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wu, Y.-K.; Ai, F.-F.; Fan, J.; Xia, Z.-P.; Liu, Y. Hierarchical Porous Polyamide 6 by Solution Foaming: Synthesis, Characterization and Properties. Polymers 2018, 10, 1310. https://doi.org/10.3390/polym10121310
Wang L, Wu Y-K, Ai F-F, Fan J, Xia Z-P, Liu Y. Hierarchical Porous Polyamide 6 by Solution Foaming: Synthesis, Characterization and Properties. Polymers. 2018; 10(12):1310. https://doi.org/10.3390/polym10121310
Chicago/Turabian StyleWang, Liang, Yu-Ke Wu, Fang-Fang Ai, Jie Fan, Zhao-Peng Xia, and Yong Liu. 2018. "Hierarchical Porous Polyamide 6 by Solution Foaming: Synthesis, Characterization and Properties" Polymers 10, no. 12: 1310. https://doi.org/10.3390/polym10121310