Chirality Construction from Preferred π-π Stacks of Achiral Azobenzene Units in Polymer: Chiral Induction, Transfer and Memory
Abstract
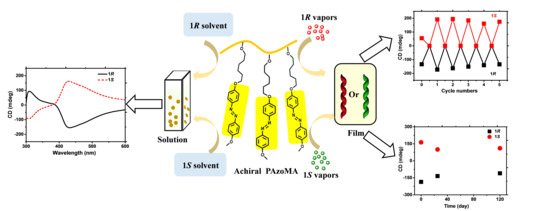
:1. Introduction
2. Experiment
2.1. Materials
2.2. Synthesis of Poly(6-[4-(4-methoxyphenylazo) phenoxy] Hexyl Methacrylate (PAzoMA)
2.3. Preparation of the Optically Active Polymer Aggregates in Solution
2.4. Preparation of the Polymer Solid Films
2.5. Chiral Induction Process of the Films
2.6. Characterization
3. Results and Discussion
3.1. Synthesis and Characterization of Side-Chain Azo-Containing Polymers
3.2. The Chiral Aggregation of the Azo-Containing Polymer in Neat Limonene Solution
3.3. Supramolecular Chirality of Polymer Solid Films
3.4. Chiral Memory Property of Azo-Containing Polymer Film
3.5. Chiral Amplification
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nafie, L.A. Physical chemistry handedness detected by microwaves. Nature 2013, 497, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J.J.L.M.; Rowan, A.E.; Nolte, R.J.M.; Sommerdijk, N.A.J.M. Chiral architectures from macromolecular building blocks. Chem. Rev. 2001, 101, 4039–4070. [Google Scholar] [CrossRef] [PubMed]
- Okoshi, K.; Sakurai, S.; Ohsawa, S.; Kumaki, J.; Yashima, E. Control of main-chain stiffness of a helical poly(phenylacetylene) by switching on and off the intramolecular hydrogen bonding through macromolecular helicity inversion. Angew. Chem. Int. Ed. 2006, 45, 8173–8176. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Debnath, S.; Javid, N.; Frederix, P.W.; Fleming, S.; Pappas, C.; Ulijn, R.V. Differential self-assembly and tunable emission of aromatic peptide bola-amphiphiles containing perylene bisimide in polar solvents including water. Langmuir 2014, 30, 7576–7584. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Jin, Y.J.; Kim, H.; Suzuki, N.; Fujiki, M.; Sakaguchi, T.; Kim, S.K.; Lee, W.E.; Kwak, G. Solvent-to-polymer chirality transfer in intramolecular stack structure. Macromolecules 2012, 45, 5379–5386. [Google Scholar] [CrossRef]
- Marinelli, F.; Sorrenti, A.; Corvaglia, V.; Leone, V.; Mancini, G. Molecular description of the propagation of chirality from molecules to complex systems: Different mechanisms controlled by hydrophobic interactions. Chem. Eur. J. 2012, 18, 14680–14688. [Google Scholar] [CrossRef] [PubMed]
- Sobczuk, A.A.; Tsuchiya, Y.; Shiraki, T.; Tamaru, S.; Shinkai, S. Creation of chiral thixotropic gels through a crown-ammonium interaction and their application to a memory-erasing recycle system. Chem. Eur. J. 2012, 18, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Akagi, K. Helical polyacetylene: Asymmetric polymerization in a chiral liquid-crystal field. Chem. Rev. 2009, 109, 5354–5401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yoshida, K.; Fujiki, M.; Zhu, X.L. Unpolarized-light-driven amplified chiroptical modulation between chiral aggregation and achiral disaggregation of an azobenzene-alt-fluorene copolymer in limonene. Macromolecules 2011, 44, 5105–5111. [Google Scholar] [CrossRef]
- Hase, Y.; Nagai, K.; Iida, H.; Maeda, K.; Ochi, N.; Sawabe, K.; Sakajiri, K.; Okoshi, K.; Yashima, E. Mechanism of helix induction in poly(4-carboxyphenyl isocyanide) with chiral amines and memory of the macromolecular helicity and its helical structures. J. Am. Chem. Soc. 2009, 131, 10719–10732. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Ichiyanagi, F.; Naito, M.; Yang, Y.G.; Fujiki, M. Chiroptical generation and inversion during the mirror-symmetry-breaking aggregation of dialkylpolysilanes due to limonene chirality. Chem. Commun. 2012, 48, 6636–6638. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, L.; Zhang, W.; Zhu, X.; Fujiki, M. Circularly polarized light with sense and wavelengths to regulate azobenzene supramolecular chirality in optofluidic medium. J. Am. Chem. Soc. 2017, 139, 13218–13226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sakamoto, T.; Nakano, T. Molecular chirality induction to an achiral pi-conjugated polymer by circularly polarized light. Chem. Commun. 2012, 48, 1871–1873. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Jiang, H.; Kohn, H.; Manaka, T.; Iwamoto, M. Control and modulation of chirality for azobenzene-substituted polydiacetylene lb films with circularly polarized light. Chem. Commun. 2009, 5627–5629. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, M.H. Chiral molecular assemblies from a novel achiral amphiphilic 2-(heptadecyl) naphtha[2,3]imidazole through interfacial coordination. J. Am. Chem. Soc. 2003, 125, 5051–5056. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.C.; Wang, T.Y.; Shi, L.; Tang, Z.Y.; Liu, M.H. Strong circularly polarized luminescence from the supramolecular gels of an achiral gelator: Tunable intensity and handedness. Chem. Sci. 2015, 6, 4267–4272. [Google Scholar] [CrossRef] [PubMed]
- Bosnich, B. Asymmetric syntheses asymmetric transformations and asymmetric inductions in an optically active solvent. J. Am. Chem. Soc. 1967, 89, 6143–6148. [Google Scholar] [CrossRef]
- Jonkheijm, P.; Miura, A.; Zdanowska, M.; Hoeben, F.J.; De Feyter, S.; Schenning, A.P.; De Schryver, F.C.; Meijer, E.W. Pi-conjugated oligo-(p-phenylenevinylene) rosettes and their tubular self-assembly. Angew. Chem. Int. Ed. 2004, 43, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Makiguchi, W.; Kobayashi, S.; Furusho, Y.; Yashima, E. Formation of a homo double helix of a conjugated polymer with carboxy groups and amplification of the macromolecular helicity by chiral amines sandwiched between the strands. Angew. Chem. Int. Ed. 2013, 52, 5275–5279. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Tamaoki, N. Chirality transfer from chiral solvents and its memory in an azobenzene derivative exhibiting photo-switchable racemization. Org. Biomol. Chem. 2011, 9, 5389–5393. [Google Scholar] [CrossRef] [PubMed]
- Green, M.M.; Khatri, C.; Peterson, N.C. A macromolecular conformational change driven by a minute chiral solvation energy. J. Am. Chem. Soc. 1993, 115, 4941–4942. [Google Scholar] [CrossRef]
- Nakashima, H.; Koe, J.R.; Torimitsu, K.; Fujiki, M. Transfer and amplification of chiral molecular information to polysilylene aggregates. J. Am. Chem. Soc. 2001, 123, 4847–4848. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Liu, Y.; Fujiki, M. Ambidextrous circular dichroism and circularly polarised luminescence from poly(9,9-di-n-decylfluorene) by terpene chirality transfer. Polym. Chem. 2010, 1, 460–469. [Google Scholar] [CrossRef]
- Kawagoe, Y.; Fujiki, M.; Nakano, Y. Limonene magic: Noncovalent molecular chirality transfer leading to ambidextrous circularly polarised luminescent pi-conjugated polymers. New J. Chem. 2010, 34, 637–647. [Google Scholar] [CrossRef]
- Fujiki, M.; Kawagoe, Y.; Nakano, Y.; Nakao, A. Mirror-symmetry-breaking in poly[(9,9-di-n-octylfluorenyl-2,7-diyl)-alt-biphenyl] (pf8p2) is susceptible to terpene chirality, achiral solvents, and mechanical stirring. Molecules 2013, 18, 7035–7057. [Google Scholar] [CrossRef] [PubMed]
- Yashima, E.; Matsushima, T.; Okamoto, Y. Chirality assignment of amines and amino alcohols based on circular dichroism induced by helix formation of a stereoregular poly((4-carboxyphenyl)acetylene) through acid-base complexation. J. Am. Chem. Soc. 1997, 119, 6345–6359. [Google Scholar] [CrossRef]
- Jiang, S.Q.; Zhao, Y.; Wang, L.; Yin, L.B.; Zhang, Z.B.; Zhu, J.; Zhang, W.; Zhu, X.L. Photocontrollable induction of supramolecular chirality in achiral side chain azo-containing polymers through preferential chiral solvation. Polym. Chem. 2015, 6, 4230–4239. [Google Scholar] [CrossRef]
- Lustig, S.R.; Everlof, G.J.; Jaycox, G.D. Stimuli-responsive polymers. 5. Azobenzene modified polyaramides containing atropisomeric binaphthyl linkages: Tuning chiroptical behavior with light and heat. Macromolecules 2001, 34, 2364–2372. [Google Scholar] [CrossRef]
- Yin, L.; Zhao, Y.; Liu, M.; Zhou, N.C.; Zhang, W.; Zhu, X.L. Induction of supramolecular chirality by chiral solvation in achiral Azo-containing polymers with different spacer lengths and push-pull electronic substituents: Where will chiral induction appear? Polym. Chem. 2017, 8, 1906–1913. [Google Scholar] [CrossRef]
- Yin, L.; Zhao, Y.; Jiang, S.Q.; Wang, L.B.; Zhang, Z.B.; Zhu, J.; Zhang, W.; Zhu, X.L. Preferential chiral solvation induced supramolecular chirality in optically inactive star Azo-containing polymers: Photocontrollability, chiral amplification and topological effects. Polym. Chem. 2015, 6, 7045–7052. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, H.; Yin, L.; Cheng, X.; Zhang, W.; Zhu, X. Chirality induction of achiral polydialkylfluorenes by chiral solvation: Odd–even and side chain length dependence. Polym. Chem. 2018, 9, 2295–2301. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K. Chirality-responsive helical polymers. Macromolecules 2008, 41, 3–12. [Google Scholar] [CrossRef]
- Rizzo, P.; Montefusco, T.; Guerra, G. Chiral optical films based on achiral chromophore guests. J. Am. Chem. Soc. 2011, 133, 9872–9877. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Shin, B.G.; Kim, J.J.; Kim, D.Y. Photoinduced supramolecular chirality in amorphous azobenzene polymer films. J. Am. Chem. Soc. 2002, 124, 3504–3505. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Natansohn, A.; Rochon, P. Photoinduced chirality in thin films of achiral polymer liquid crystals containing azobenzene chromophores. Macromolecules 2004, 37, 6801–6805. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Chen, X.; Cheng, Z.; Wu, J.; Zhu, J.; Zhu, X. Synthesis of novel three-arm star azo side-chain liquid crystalline polymer via atrp and photoinduced surface relief gratings. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 777–789. [Google Scholar] [CrossRef]
- Han, D.H.; Tong, X.; Zhao, Y.; Galstian, T.; Zhao, Y. Cyclic azobenzene-containing side-chain liquid crystalline polymers: Synthesis and topological effect on mesophase transition, order, and photoinduced birefringence. Macromolecules 2010, 43, 3664–3671. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.G. Synthesis and photoresponsive properties of two liquid crystalline polymers bearing branched azobenzene-containing side chains. Polym. Chem. 2013, 4, 5108–5118. [Google Scholar] [CrossRef]
- Wang, T.J.; Li, X.; Dong, Z.J.; Huang, S.; Yu, H.F. Vertical orientation of nanocylinders in liquid-crystalline block copolymers directed by light. ACS Appl. Mater. Interfaces 2017, 9, 24864–24872. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Rahirn, N.A.A.; Xia, Y.J.; Fujiki, M.; Song, B.; Zhang, Z.B.; Zhang, W.; Zhu, X.L. Supramolecular chirality in achiral polyfluorene: Chiral gelation, memory of chirality, and chiral sensing property. Macromolecules 2016, 49, 3214–3221. [Google Scholar] [CrossRef]






| Entry | Ratio a | Conv. b (%) | Mn(th)c (g mol−1) | Mn(GPC)d (g mol−1) | Mw/Mnd |
|---|---|---|---|---|---|
| PAzoMA1 | 20:1:1:1 | 69.5 | 5500 | 7400 | 1.20 |
| PAzoMA2 | 70:1:1:1 | 53.4 | 14,800 | 12,400 | 1.14 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, T.; Yin, L.; Cheng, X.; Zhao, Y.; Hou, W.; Zhang, W.; Zhu, X. Chirality Construction from Preferred π-π Stacks of Achiral Azobenzene Units in Polymer: Chiral Induction, Transfer and Memory. Polymers 2018, 10, 612. https://doi.org/10.3390/polym10060612
Miao T, Yin L, Cheng X, Zhao Y, Hou W, Zhang W, Zhu X. Chirality Construction from Preferred π-π Stacks of Achiral Azobenzene Units in Polymer: Chiral Induction, Transfer and Memory. Polymers. 2018; 10(6):612. https://doi.org/10.3390/polym10060612
Chicago/Turabian StyleMiao, Tengfei, Lu Yin, Xiaoxiao Cheng, Yin Zhao, Wenjie Hou, Wei Zhang, and Xiulin Zhu. 2018. "Chirality Construction from Preferred π-π Stacks of Achiral Azobenzene Units in Polymer: Chiral Induction, Transfer and Memory" Polymers 10, no. 6: 612. https://doi.org/10.3390/polym10060612