Degradation of Silicone Rubbers as Sealing Materials for Proton Exchange Membrane Fuel Cells under Temperature Cycling
Abstract
:1. Introduction
2. Experimental
2.1. Material and Test Environments
2.2. Aging and Characterization Methods
3. Results and Discussion
3.1. Weight Change
3.2. Hardness Change
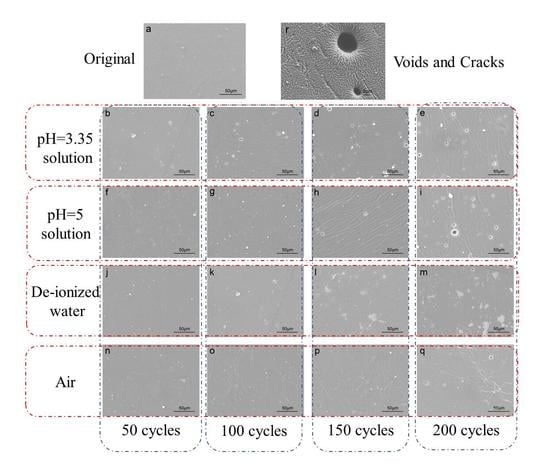
3.3. Scanning Electron Microscopy
3.4. ATR-FTIR
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wan, Z.H.; Zhong, Q.; Liu, S.F.; Jin, A.P.; Chen, Y.N.; Tan, J.T.; Pan, M. Determination of oxygen transport resistance in gas diffusion layer for polymer electrolyte fuel cells. Int. J. Energy Res. 2018, 42, 2225–2233. [Google Scholar] [CrossRef]
- Srinivasan, S. Fuel Cells: From Fundamentals to Applications; Springer: New York, NY, USA, 2006. [Google Scholar]
- Tsai, M.-J.; Hsieh, T.-H.; Wang, Y.-Z.; Ho, K.-S.; Chang, C.-Y. Microwave assisted reduction of pt-catalyst by N-phenyl-p-phenylenediamine for proton exchange membrane fuel cells. Polymers 2017, 9, 104. [Google Scholar] [CrossRef]
- Brandon, N.P.; Skinner, S.; Steele, B.C.H. Recent advances in materials for fuel cells. Annu. Rev. Mater. Res. 2003, 33, 183–213. [Google Scholar] [CrossRef]
- Basuli, U.; Jose, J.; Lee, R.H.; Yoo, Y.H.; Jeong, K.-U.; Ahn, J.-H.; Nah, C. Properties and degradation of the gasket component of a proton exchange membrane fuel cell—A review. J. Nanosci. Nanotechnol. 2012, 12, 7641–7657. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, F.A.; Dam, V.A.T.; Janssen, G.J.M. Review: Durability and degradation issues of PEM fuel cell components. Fuel Cells 2008, 8, 3–22. [Google Scholar] [CrossRef]
- Chang, H.; Wan, Z.; Chen, X.; Wan, J.; Luo, L.; Zhang, H.; Shu, S.; Tu, Z. Temperature and humidity effect on aging of silicone rubbers as sealing materials for proton exchange membrane fuel cell applications. Appl. Therm. Eng. 2016, 104, 472–478. [Google Scholar] [CrossRef]
- Lin, C.-W.; Chien, C.-H.; Tan, J.; Chao, Y.J.; Van Zee, J.W. Chemical degradation of five elastomeric seal materials in a simulated and an accelerated PEM fuel cell environment. J. Power Sources 2011, 196, 1955–1966. [Google Scholar] [CrossRef]
- Ye, D.-H.; Zhan, Z.-G. A review on the sealing structures of membrane electrode assembly of proton exchange membrane fuel cells. J. Power Sources 2013, 231, 285–292. [Google Scholar] [CrossRef]
- Pehlivan-Davis, S.; Clarke, J.; Armour, S. Comparison of accelerated aging of silicone rubber gasket material with aging in a fuel cell environment. J. Appl. Polym. Sci. 2013, 129, 1446–1454. [Google Scholar] [CrossRef]
- Cleghorn, S.J.C.; Mayfield, D.K.; Moore, D.A.; Moore, J.C.; Rusch, G.; Sherman, T.W.; Sisofo, N.T.; Beuscher, U. A polymer electrolyte fuel cell life test: 3 years of continuous operation. J. Power Sources 2006, 158, 446–454. [Google Scholar] [CrossRef]
- Bhargava, S.; O’Leary, K.A.; Jackson, T.C.; Lakshmanan, B. Durability testing of silicone materials for proton exchange membrane fuel cell use. Rubber Chem. Technol. 2013, 86, 28–37. [Google Scholar] [CrossRef]
- Cui, T.; Chao, Y.J.; Chen, X.M.; Van Zee, J.W. Effect of water on life prediction of liquid silicone rubber seals in polymer electrolyte membrane fuel cell. J. Power Sources 2011, 196, 9536–9543. [Google Scholar] [CrossRef]
- Li, X.; An, Y.H.; Wu, Y.D.; Song, Y.C.; Chao, Y.J.; Chien, C.H. Microindentation test for assessing the mechanical properties of cartilaginous tissues. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 80, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, A.H.; Kim, J.K.; Kim, S.J. Life time prediction of rubber gasket for fuel cell through its acid-aging characteristics. Macromol. Res. 2007, 15, 315–323. [Google Scholar] [CrossRef]
- Li, G.; Tan, J.; Gong, J. Degradation of the elastomeric gasket material in a simulated and four accelerated proton exchange membrane fuel cell environments. J. Power Sources 2012, 205, 244–251. [Google Scholar] [CrossRef]
- Li, G.; Tan, J.; Gong, J. Chemical aging of the silicone rubber in a simulated and three accelerated proton exchange membrane fuel cell environments. J. Power Sources 2012, 217, 175–183. [Google Scholar] [CrossRef]
- Cui, T.; Lin, C.W.; Chien, C.H.; Chao, Y.J.; Van Zee, J.W. Service life estimation of liquid silicone rubber seals in polymer electrolyte membrane fuel cell environment. J. Power Sources 2011, 196, 1216–1221. [Google Scholar] [CrossRef]
- Lin, C.-W.; Chien, C.-H.; Tan, J.; Chao, Y.-J.; Van Zee, J.W. Dynamic mechanical characteristics of five elastomeric gasket materials aged in a simulated and an accelerated PEM fuel cell environment. Int. J. Hydrog. Energy 2011, 36, 6756–6767. [Google Scholar] [CrossRef]
- Curtin, D.E.; Lousenberg, R.D.; Henry, T.J.; Tangeman, P.C.; Tisack, M.E. Advanced materials for improved pemfc performance and life. J. Power Sources 2004, 131, 41–48. [Google Scholar] [CrossRef]
- Graiver, D.; Farminer, K.W.; Narayan, R. A review of the fate and effects of silicones in the environment. J. Polym. Environ. 2003, 11, 129–136. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, I.-H. Characteristics of surface wettability and hydrophobicity and recovery ability of EPDM rubber and silicone rubber for polymer insulators. J. Appl. Polym. Sci. 2001, 79, 2251–2257. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Q.; Tu, Z.; Tu, W.; Wan, Z.; Pan, M.; Zhang, H. Degradation of silicone rubbers with different hardness in various aqueous solutions. Polym. Degrad. Stab. 2014, 109, 122–128. [Google Scholar] [CrossRef]
- Schulze, M.; Knöri, T.; Schneider, A.; Gülzow, E. Degradation of sealings for PEFC test cells during fuel cell operation. J. Power Sources 2004, 127, 222–229. [Google Scholar] [CrossRef]
- Tan, J.; Chao, Y.J.; Yang, M.; Lee, W.-K.; Van Zee, J.W. Chemical and mechanical stability of a silicone gasket material exposed to PEM fuel cell environment. Int. J. Hydrog. Energy 2011, 36, 1846–1852. [Google Scholar] [CrossRef]
- Tan, J.; Chao, Y.J.; Li, X.; Van Zee, J.W. Microindentation test for assessing the mechanical properties of silicone rubber exposed to a simulated polymer electrolyte membrane fuel cell environment. J. Fuel Cell Sci. Technol. 2009, 6, 041017. [Google Scholar] [CrossRef]
- Tan, J.; Chao, Y.J.; Yang, M.; Williams, C.T.; Van Zee, J.W. Degradation characteristics of elastomeric gasket materials in a simulated PEM fuel cell environment. J. Mater. Eng. Perform. 2008, 17, 785–792. [Google Scholar] [CrossRef]
- Tan, J.; Chao, Y.J.; Van Zee, J.W.; Lee, W.K. Degradation of elastomeric gasket materials in PEM fuel cells. Mater. Sci. Eng. A 2007, 445–446, 669–675. [Google Scholar] [CrossRef]
- Tan, J.; Chao, Y.J.; Li, X.; Van Zee, J.W. Degradation of silicone rubber under compression in a simulated PEM fuel cell environment. J. Power Sources 2007, 172, 782–789. [Google Scholar] [CrossRef]
- Cui, T.; Chao, Y.J.; Van Zee, J.W. Sealing force prediction of elastomeric seal material for PEM fuel cell under temperature cycling. Int. J. Hydrog. Energy 2014, 39, 1430–1438. [Google Scholar] [CrossRef]
- Cui, T.; Chao, Y.J.; Van Zee, J.W. Thermal stress development of liquid silicone rubber seal under temperature cycling. Polym. Test. 2013, 32, 1202–1208. [Google Scholar] [CrossRef]
- Tu, Z.; Zhang, H.; Luo, Z.; Liu, J.; Wan, Z.; Pan, M. Evaluation of 5 kw proton exchange membrane fuel cell stack operated at 95 °C under ambient pressure. J. Power Sources 2013, 222, 277–281. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Zhang, J.; Tang, Y.; Song, C.; Navessin, T.; Shi, Z.; Song, D.; Wang, H.; Wilkinson, D.P.; et al. High temperature PEM fuel cells. J. Power Sources 2006, 160, 872–891. [Google Scholar] [CrossRef]
- Torchio, M.F.; Santarelli, M.G.; Nicali, A. Experimental analysis of the CHP performance of a PEMFC stack by a 24 factorial design. J. Power Sources 2005, 149, 33–43. [Google Scholar] [CrossRef]
- Arsalis, A.; Nielsen, M.P.; Kær, S.K. Modeling and optimization of a 1 kWe HT-PEMFC-based micro-CHP residential system. Int. J. Hydrog. Energy 2012, 37, 2470–2481. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Gallorini, F.; Ottaviano, A. Dynamic analysis of PEMFC-based CHP systems for domestic application. Appl. Energy 2012, 91, 13–28. [Google Scholar] [CrossRef]
- Gittleman, C. Automotive Perspective on PEM Evaluation. In Proceedings of the High Temperature Membrane Working Group Meeting, Washington, DC, USA, 18 May 2009. [Google Scholar]
- Wu, W. Analysis on the development environment of Shanghai hydrogen fuel cell vehicle industry. Shanghai Auto 2014, 9, 29–33. [Google Scholar]
- Meng, H.; Ruan, B. Numerical studies of cold-start phenomena in PEM fuel cells: A review. Int. J. Energy Res. 2011, 35, 2–14. [Google Scholar] [CrossRef]
- Yoshimura, N. Electrical and environmental aging of silicone rubber use in outdoor insulation. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 632–650. [Google Scholar] [CrossRef]
- Bao, Y.W.; Wang, W.; Zhou, Y.C. Investigation of the relationship between elastic modulus and hardness based on depth-sensing indentation measurements. Acta Mater. 2004, 52, 5397–5404. [Google Scholar] [CrossRef]
- Delor-Jestin, F.; Tomer, N.S.; Singh, R.P.; Lacoste, J. Characterization of polydimethylsiloxane rubber upon photochemical, thermal, salf-fog ageings and exposure to acid vapours. e-Polymers 2006, 6. [Google Scholar] [CrossRef]
- Hu, Y.; Mei, R.; An, Z.; Zhang, J. Influence of inorganic fillers on the mechanical properties of silicone rubber. In Proceedings of the Beijing Adhesion Society Twentieth Annual Meeting and Conference on Technical Development of Adhesives, Beijing, China, 19 October 2011. [Google Scholar]







| Area (mm2) | 4000 |
|---|---|
| Length (mm) | 100 |
| Width (mm) | 40 |
| Thickness (mm) | 0.5 |
| Testing Cycle | Testing Environment (%) | |||
|---|---|---|---|---|
| pH = 3.35 | pH = 5 | pH = 7 | AIR | |
| 50th | 0.0060 | 0.0050 | 0.0050 | 0.0024 |
| 100th | 0.0037 | 0.0033 | 0.0028 | 0.0014 |
| 150th | 0.0031 | 0.0030 | 0.0020 | 0.0018 |
| 200th | 0.0032 | 0.0025 | 0.0019 | 0.0017 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Chen, B.; Yan, Y.; Chen, Y.; Pan, M. Degradation of Silicone Rubbers as Sealing Materials for Proton Exchange Membrane Fuel Cells under Temperature Cycling. Polymers 2018, 10, 522. https://doi.org/10.3390/polym10050522
Wu F, Chen B, Yan Y, Chen Y, Pan M. Degradation of Silicone Rubbers as Sealing Materials for Proton Exchange Membrane Fuel Cells under Temperature Cycling. Polymers. 2018; 10(5):522. https://doi.org/10.3390/polym10050522
Chicago/Turabian StyleWu, Fan, Ben Chen, Yizhi Yan, Yanan Chen, and Mu Pan. 2018. "Degradation of Silicone Rubbers as Sealing Materials for Proton Exchange Membrane Fuel Cells under Temperature Cycling" Polymers 10, no. 5: 522. https://doi.org/10.3390/polym10050522