Plant Secondary Metabolite-Derived Polymers: A Potential Approach to Develop Antimicrobial Films
Abstract
:1. Introduction
- The challenge of bacterial adhesion, biofilms formation, and medical device-associated infections.
- The retention of inherent antimicrobial activity of sustainable monomers, e.g., plant secondary metabolites within solid polymers with the aim of applying them as bioactive coatings.
2. Microbial Contamination
2.1. Bacterial Adhesion
2.2. Biofilm Formation
2.3. The Impact of Biofilm Formation in the Healthcare Environment
- Overview on plant extracts:
3. The Antibacterial Activities of PSMs
3.1. The Antibacterial Mechanisms of PSMs
3.2. Sustainable Polymers from Bioactive Essential Oils
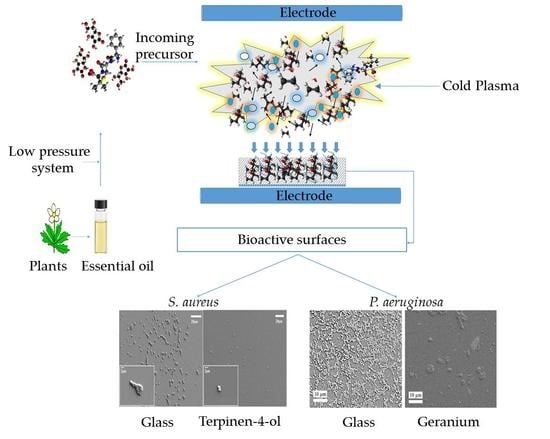
3.3. Plasma-Assisted Fabrication of PSMs
3.3.1. Terpinen-4-ol
3.3.2. Carvone
3.3.3. Eucalyptol
3.3.4. Geranium
3.4. Properties of PSM-Derived Polymers
- Essential oils variations:
4. Challenges
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanborn, L.W.R. The relation of surface contamination to the transmission of disease. Am. J. Public Health Nations Health 1963, 53, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: The case for hospital cleaning. Lancet Infect. Dis. 2008, 8, 101–113. [Google Scholar] [CrossRef]
- Knetsch, M.L.W.; Koole, L.H. New strategies in the development of antimicrobial coatings: The example of increasing usage of silver and silver nanoparticles. Polymers 2011, 3, 340–366. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Yeung, A.; Yeung, K.W.; Kao, R.Y.; Wu, G.; Hu, T.; Xu, Z.; Chu, P.K. Plasma-modified biomaterials for self-antimicrobial applications. ACS Appl. Mater. Interfaces 2011, 3, 2851–2860. [Google Scholar] [CrossRef] [PubMed]
- Edmiston, C.E., Jr.; McBain, A.J.; Roberts, C.; Leaper, D. Clinical and microbiological aspects of biofilm-associated surgical site infections. In Biofilm-Based Healthcare-Associated Infections; Springer: International Publishing: Cham, Switzerland, 2015; pp. 47–67. [Google Scholar]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial Adherence and Biofilm Formation on Medical Implants: A Review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta Biomembr. 2016, 1858, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.; Naugolny, A.; Feldman, M.; Herzog, I.M.; Fridman, M.; Cohen, Y. Cationic pillararenes potently inhibit biofilm formation without affecting bacterial growth and viability. J. Am. Chem. Soc. 2016, 138, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Mingeot-Leclercq, M.-P.; Décout, J.-L. Bacterial lipid membranes as promising targets to fight antimicrobial resistance, molecular foundations and illustration through the renewal of aminoglycoside antibiotics and emergence of amphiphilic aminoglycosides. MedChemComm 2016, 7, 586–611. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Woon, S.-A.; Fisher, D. Antimicrobial agents–optimising the ecological balance. BMC Med. 2016, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Singh, R. Antibiotic resistance in food lactic acid bacteria—A review. Int. J. Food Microbiol. 2005, 105, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Willers, C.; Wentzel, J.F.; Plessis, L.H.d.; Gouws, C.; Hamman, J.H. Efflux as a mechanism of antimicrobial drug resistance in clinical relevant microorganisms: The role of efflux inhibitors. Expert Opin. Ther. Targets 2017, 21, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Concia, E.; Cristini, F.; de Rosa, F.G.; Esposito, S.; Menichetti, F.; Petrosillo, N.; Tumbarello, M.; Venditti, M.; Viale, P.; et al. Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin. Microbiol. Infect. 2016, 22, S27–S36. [Google Scholar] [CrossRef]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, C.A.; Pendleton, S.J.; Crandall, P.G.; Ricke, S.C. Potential of plant essential oils and their components in animal agriculture–in vitro studies on antibacterial mode of action. Front. Vet. Sci. 2015, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Murbach Teles Andrade, B.F.; Nunes Barbosa, L.; da Silva Probst, I.; Fernandes Júnior, A. Antimicrobial activity of essential oils. J. Essent. Oil Res. 2014, 26, 34–40. [Google Scholar] [CrossRef]
- Altenstetter, C. Global and local dynamics: The regulation of medical technologies in the European Union, Japan and the United States. Presented to panel 6E Context and Regulatory Design, Third Biennial Conference ‘Regulation in the Age of Crisis’, Dublin, Ireland, 17–19 June 2010. [Google Scholar]
- Méndez-Vilas, A.; Díaz, J. Microscopy: Science, Technology, Applications and Education; Formatex Research Center: Badajoz, Spain, 2010. [Google Scholar]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Chandki, R.; Banthia, P.; Banthia, R. Biofilms: A microbial home. J. Indian Soc. Periodontol. 2011, 15, 111–114. [Google Scholar] [PubMed]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E.W. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Harapanahalli, A.K.; Busscher, H.J.; Norde, W.; van der Mei, H.C. Nanoscale cell wall deformation impacts long-range bacterial adhesion forces on surfaces. Appl. Environ. Microbiol. 2014, 80, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Scheuerman, T.R.; Camper, A.K.; Hamilton, M.A. Effects of substratum topography on bacterial adhesion. J. Colloid Interface Sci. 1998, 208, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.J.; Ivanova, E.P. Superhydrophobic Surfaces; Elsevier: New York, NY, USA, 2015. [Google Scholar]
- Boland, T.; Latour, R.A.; Stutzenberger, F.J. Molecular basis of bacterial adhesion. In Handbook of Bacterial Adhesion; Springer: Berlin/Heidelberg, Germany, 2000; pp. 29–41. [Google Scholar]
- Basak, S.; Rajurkar, M.N.; Attal, R.O.; Mallick, S.K. Biofilms: A challenge to medical fraternity in infection control. Infect. Control 2013, 57. [Google Scholar] [CrossRef]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.A.; Reizian, M.A.; Chapman, M.R. Spatial clustering of the curlin secretion lipoprotein requires curli fiber assembly. J. Bacteriol. 2009, 191, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.; Savage, D.C. Bacterial Adhesion: Mechanisms and Physiological Significance; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Moss, J.A.; Nocker, A.; Lepo, J.E.; Snyder, R.A. Stability and change in estuarine biofilm bacterial community diversity. Appl. Environ. Microbiol. 2006, 72, 5679–5688. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Douglas, L.J. Candida biofilms and their role in infection. Trends Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Ma, L.; Conover, M.; Lu, H.; Parsek, M.R.; Bayles, K.; Wozniak, D.J. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 2009, 5, e1000354. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Karig, D.; Kumar, A.; Ardekani, A. Interplay of physical mechanisms and biofilm processes: Review of microfluidic methods. Lab Chip 2015, 15, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Sharafat, I.; Saeed, D.K.; Yasmin, S.; Imran, A.; Zafar, Z.; Hameed, A.; Ali, N. Interactive effect of trivalent iron on activated sludge digestion and biofilm structure in attached growth reactor of waste tire rubber. Environ. Technol. 2017, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Usman, J.; Kaleem, F.; Omair, M.; Khalid, A.; Iqbal, M. Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz. J. Infect. Dis. 2011, 15, 305–311. [Google Scholar] [CrossRef]
- Nguyen, S.H.; Webb, H.K.; Crawford, R.J.; Ivanova, E.P. Natural antibacterial surfaces. In Antibacterial Surfaces; Springer: Berlin/Heidelberg, Germany, 2015; pp. 9–26. [Google Scholar]
- Jackson, D.W.; Suzuki, K.; Oakford, L.; Simecka, J.W.; Hart, M.E.; Romeo, T. Biofilm formation and dispersal under the influence of the global regulator csra of Escherichia coli. J. Bacteriol. 2002, 184, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontology 2000 2002, 28, 12–55. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilm formation: A clinically relevant microbiological process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Derlon, N.; Coufort-Saudejaud, C.; Queinnec, I.; Paul, E. Growth limiting conditions and denitrification govern extent and frequency of volume detachment of biofilms. Chem. Eng. J. 2013, 218, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Barraud, N.; Hassett, D.J.; Hwang, S.-H.; Rice, S.A.; Kjelleberg, S.; Webb, J.S. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 7344–7353. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.-A.; Schiesser, C.H. Heteroorganic molecules and bacterial biofilms: Controlling biodeterioration of cultural heritage. Org. Chem. 2017, 180–222. [Google Scholar]
- Fish, K.E.; Osborn, A.M.; Boxall, J. Characterising and understanding the impact of microbial biofilms and the extracellular polymeric substance (EPS) matrix in drinking water distribution systems. Environ. Sci. Water Res. Technol. 2016, 2, 614–630. [Google Scholar] [CrossRef]
- Kaplan, J.Á. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Parsek, M.R. Controlling the connections of cells to the biofilm matrix. J. Bacteriol. 2016, 198, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Rasamiravaka, T.; Labtani, Q.; Duez, P.; El Jaziri, M. The formation of biofilms by Pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. BioMed Res. Int. 2015, 2015, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bryers, J.D. Medical biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Device-Related Infections. Available online: http://www.infectioncontroltoday.com/articles/2006/11/device-related-infections.aspx (accessed on 3 May 2018).
- Pradeep, K.S.; Easwer, H.; Maya, N.A. Multiple drug resistant bacterial biofilms on implanted catheters—A reservoir of infection. J. Assoc. Phys. India 2013, 61, 702–707. [Google Scholar]
- Klevens, R.M.; Edwards, J.R.; Gaynes, R.; System, N.N.I.S. The impact of antimicrobial-resistant, health care–associated infections on mortality in the United States. Clin. Infect. Dis. 2008, 47, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; De Prijck, K.; Nailis, H.; Nelis, H.J. Prevention of Candida albicans biofilm formation. Open Mycol. J. 2011, 5, 9–20. [Google Scholar] [CrossRef]
- Deorukhkar, S.C.; Saini, S. Why Candida species have emerged as important nosocomial pathogens? Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 533–545. [Google Scholar] [CrossRef]
- Sanclement, J.A.; Webster, P.; Thomas, J.; Ramadan, H.H. Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis. Laryngoscope 2005, 115, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Martínez, J.P.; López-Ribot, J.L. Candida biofilms on implanted biomaterials: A clinically significant problem. FEMS Yeast Res. 2006, 6, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Jabra-Rizk, M.A.; Kong, E.F.; Tsui, C.; Nguyen, M.H.; Clancy, C.J.; Fidel, P.L.; Noverr, M. Candida albicans pathogenesis: Fitting within the host-microbe damage response framework. Infect. Immun. 2016, 84, 2724–2739. [Google Scholar] [CrossRef] [PubMed]
- Alhede, M.; Kragh, K.N.; Qvortrup, K.; Allesen-Holm, M.; van Gennip, M.; Christensen, L.D.; Jensen, P.Ø.; Nielsen, A.K.; Parsek, M.; Wozniak, D. Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. PLoS ONE 2011, 6, e27943. [Google Scholar] [CrossRef] [PubMed]
- Wagner, V.E.; Iglewski, B.H. P. aeruginosa biofilms in CF infection. Clin. Rev. Allergy Immunol. 2008, 35, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Firenzuoli, F.; Jaitak, V.; Horvath, G.; Bassolé, I.H.N.; Setzer, W.N.; Gori, L. Essential oils: New perspectives in human health and wellness. Evid.-Based Complement. Altern. Med. 2014, 2014, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferth, T. Antibacterial Activity and Anticancer Activity of Rosmarinus officinalis L. Essential Oil Compared to That of Its Main Components. Molecules 2012, 17, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Stupar, M.; Grbić, M.L.; Džamić, A.; Unković, N.; Ristić, M.; Jelikić, A.; Vukojević, J. Antifungal activity of selected essential oils and biocide benzalkonium chloride against the fungi isolated from cultural heritage objects. S. Afr. J. Bot. 2014, 93, 118–124. [Google Scholar] [CrossRef]
- Rotolo, V.; Barresi, G.; Di Carlo, E.; Giordano, A.; Lombardo, G.; Crimi, E.; Costa, E.; Bruno, M.; Palla, F. Plant extracts as green potential strategies to control the biodeterioration of cultural heritage. Int. J. Conserv. Sci. 2016, 2, 839–846. [Google Scholar]
- Borrego, S.; Valdés, O.; Vivar, I.; Lavin, P.; Guiamet, P.; Battistoni, P.; Gómez de Saravia, S.; Borges, P. Essential oils of plants as biocides against microorganisms isolated from cuban and argentine documentary heritage. ISRN Microbiol. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid.-Based Complement. Altern. Med. 2016, 2016, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Eze, U.A. In vitro antimicrobial activity of essential oils from the lamiaceae and rutaceae plant families against β lactamse-producing clinical isolates of Moraxella Catarrhalis. EC Pharm. Sci. 2016, 2, 325–337. [Google Scholar]
- Kateryna, B.; Olha, B.; Igor, L.; Shuyan, X.; Elena, P.I.; Michael, K.; Kostya, O. Plasma-potentiated small molecules—Possible alternative to antibiotics? Nano Futur. 2017, 1, 025002. [Google Scholar]
- Silva, N.; Fernandes Júnior, A. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 402–413. [Google Scholar] [CrossRef]
- Hammami, I.; Triki, M.A.; Rebai, A. Chemical compositions, antibacterial and antioxidant activities of essential oil and various extracts of Geranium sanguineum L. Flowers. Sch. Res. Libr. 2011, 3, 135–144. [Google Scholar]
- Hui, L.; He, L.; Huan, L.; XiaoLan, L.; AiGuo, Z. Chemical composition of Lavender essential oil and its antioxidant activity and inhibition against rhinitis-related bacteria. Afr. J. Microbiol. Res. 2010, 4, 309–313. [Google Scholar]
- Glinel, K.; Thebault, P.; Humblot, V.; Pradier, C.-M.; Jouenne, T. Antibacterial surfaces developed from bio-inspired approaches. Acta Biomater. 2012, 8, 1670–1684. [Google Scholar] [CrossRef] [PubMed]
- Hierro, I.; Valero, A.; Perez, P.; Gonzalez, P.; Cabo, M.; Montilla, M.; Navarro, M. Action of different monoterpenic compounds against Anisakis simplex s.l. L3 larvae. Phytomedicine 2004, 11, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Bigos, M.; Wasiela, M.; Kalemba, D.; Sienkiewicz, M. Antimicrobial activity of geranium oil against clinical strains of Staphylococcus aureus. Molecules 2012, 17, 10276–10291. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Henika, P.R.; Levin, C.E.; Mandrell, R.E. Antibacterial activities of plant essential oils and their components against Escherichia coli O157:H7 and Salmonella enterica in apple juice. J. Agric. Food Chem. 2004, 52, 6042–6048. [Google Scholar] [CrossRef] [PubMed]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals; Elsevier Health Sciences: New York, NY, USA, 2013. [Google Scholar]
- Sun, L.-M.; Zhang, C.-L.; Li, P. Characterization, antibiofilm, and mechanism of action of novel peg-stabilized lipid nanoparticles loaded with terpinen-4-ol. J. Agric. Food Chem. 2012, 60, 6150–6156. [Google Scholar] [CrossRef] [PubMed]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Faid, M.; Bakhy, K.; Anchad, M.; Tantaoui-Elaraki, A. Almond paste: Physicochemical and microbiological characterization and preservation with sorbic acid and cinnamon. J. Food Prot. 1995, 58, 547–550. [Google Scholar] [CrossRef]
- Maruzzella, J.C.; Sicurella, N.A. Antibacterial activity of essential oil vapors. J. Pharm. Sci. 1960, 49, 692–694. [Google Scholar] [CrossRef]
- Maruzzella, J.C.; Chiaramonte, J.S.; Garofalo, M.M. Effects of vapors of aromatic chemicals on fungi. J. Pharm. Sci. 1961, 50, 665–668. [Google Scholar] [CrossRef]
- Akthar, M.S.; Degaga, B.; Azam, T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: A review. Issues Biol. Sci. Pharm. Res. 2014, 2, 1–7. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Kets, E.; Smid, E. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar] [PubMed]
- Ultee, A.; Smid, E.J. Influence of Carvacrol on growth and toxin production by Bacillus cereus. Int. J. Food Microbiol. 2001, 64, 373–378. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Gustafson, J.; Liew, Y.; Chew, S.; Markham, J.; Bell, H.; Wyllie, S.; Warmington, J. Effects of tea tree oil on Escherichia coli. Lett. Appl. Microbiol. 1998, 26, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Szabó, M.Á.; Varga, G.Z.; Hohmann, J.; Schelz, Z.; Szegedi, E.; Amaral, L.; Molnár, J. Inhibition of quorum-sensing signals by essential oils. Phytother. Res. 2010, 24, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic plants as a source of bioactive compounds. Agriculture 2012, 2, 228–243. [Google Scholar] [CrossRef]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of Carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Ćavar, S.; Maksimović, M.; Vidic, D.; Parić, A. Chemical composition and antioxidant and antimicrobial activity of essential oil of Artemisia annua L. from Bosnia. Ind. Crops Prod. 2012, 37, 479–485. [Google Scholar] [CrossRef]
- Stoica, P.; Chifiriuc, M.C.; Râpă, M.; Bleotu, C.; Lungu, L.; Vlad, G.; Grigore, R.; Berteșteanu, Ș.; Stavropoulou, E.; Lazăr, V. Fabrication, characterization and bioevaluation of novel antimicrobial composites based on polycaprolactone, chitosan and essential oils. Rom. Biotechnol. Lett. 2015, 20, 10521–10535. [Google Scholar]
- Balat, M.; Balat, M. Political, economic and environmental impacts of biomass-based hydrogen. Int. J. Hydrog. Energy 2009, 34, 3589–3603. [Google Scholar] [CrossRef]
- Balzani, V.; Armaroli, N. Energy for a Sustainable World: From the Oil Age to a Sun-Powered Future; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Winnacker, M.; Rieger, B. Recent progress in sustainable polymers obtained from cyclic terpenes: Synthesis, properties, and application potential. ChemSusChem 2015, 8, 2455–2471. [Google Scholar] [CrossRef] [PubMed]
- Saggiorato, A.G.; Gaio, I.; Treichel, H.; de Oliveira, D.; Cichoski, A.J.; Cansian, R.L. Antifungal activity of basil essential oil (Ocimum basilicum L.): Evaluation in vitro and on an italian-type sausage surface. Food Bioprocess Technol. 2012, 5, 378–384. [Google Scholar] [CrossRef]
- Teramoto, Y.; Nishio, Y. Cellulose diacetate-graft-poly(lactic acid)s: Synthesis of wide-ranging compositions and their thermal and mechanical properties. Polymer 2003, 44, 2701–2709. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA–PHB–Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Robertson, M.L.; Paxton, J.M.; Hillmyer, M.A. Tough blends of polylactide and castor oil. ACS Appl. Mater. Interfaces 2011, 3, 3402–3410. [Google Scholar] [CrossRef] [PubMed]
- Morschbacker, A. Bio-ethanol based ethylene. Polym. Rev. 2009, 49, 79–84. [Google Scholar] [CrossRef]
- Mathers, R.T.; Damodaran, K.; Rendos, M.G.; Lavrich, M.S. Functional hyperbranched polymers using ring-opening metathesis polymerization of dicyclopentadiene with monoterpenes. Macromolecules 2009, 42, 1512–1518. [Google Scholar] [CrossRef]
- Quilter, H.C.; Hutchby, M.; Davidson, M.G.; Jones, M.D. Polymerisation of a terpene-derived lactone: A bio-based alternative to ε-caprolactone. Polym. Chem. 2017, 8, 833–837. [Google Scholar] [CrossRef]
- Gandini, A. The irruption of polymers from renewable resources on the scene of macromolecular science and technology. Green Chem. 2011, 13, 1061–1083. [Google Scholar] [CrossRef]
- Wilbon, P.A.; Chu, F.; Tang, C. Progress in renewable polymers from natural terpenes, terpenoids, and rosin. Macromol. Rapid Commun. 2013, 34, 8–37. [Google Scholar] [CrossRef] [PubMed]
- Shit, S.C.; Shah, P.M. Edible polymers: Challenges and opportunities. J. Polym. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Modjinou, T.; Versace, D.-L.; Abbad-Andallousi, S.; Bousserrhine, N.; Babinot, J.; Langlois, V.; Renard, E. Antibacterial networks based on isosorbide and linalool by photoinitiated process. ACS Sustain. Chem. Eng. 2015, 3, 1094–1100. [Google Scholar] [CrossRef]
- Chen, Y.; Wilbon, P.A.; Chen, Y.P.; Zhou, J.; Nagarkatti, M.; Wang, C.; Chu, F.; Decho, A.W.; Tang, C. Amphipathic antibacterial agents using cationic methacrylic polymers with natural rosin as pendant group. RSC Adv. 2012, 2, 10275–10282. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.-H.; Feng, K.; Liu, F.-J.; Lou, W.-Y.; Li, N.; Zong, M.-H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Avila-Sosa, R.; Palou, E.; Jiménez Munguía, M.T.; Nevárez-Moorillón, G.V.; Navarro Cruz, A.R.; López-Malo, A. Antifungal activity by vapor contact of essential oils added to amaranth, chitosan, or starch edible films. Int. J. Food Microbiol. 2012, 153, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A. Quince seed mucilage films incorporated with oregano essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocoll. 2014, 36, 9–19. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with thymus moroderi or thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Edible coatings as encapsulating matrices for bioactive compounds: A review. J. Food Sci. Technol. 2014, 51, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Sessa, M.; Ferrari, G.; Donsì, F. Novel edible coating containing essential oil nanoemulsions to prolong the shelf life of vegetable products. Chem. Eng. Trans. 2015, 43, 55–60. [Google Scholar]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Nanoencapsulation of zataria multiflora essential oil preparation and characterization with enhanced antifungal activity for controlling Botrytis cinerea, the causal agent of gray mould disease. Innov. Food Sci. Emerg. Technol. 2015, 28, 73–80. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Antimicrobial activity of alginate/clay nanocomposite films enriched with essential oils against three common foodborne pathogens. Food Control 2014, 36, 1–7. [Google Scholar] [CrossRef]
- Severino, R.; Ferrari, G.; Vu, K.D.; Donsì, F.; Salmieri, S.; Lacroix, M. Antimicrobial effects of modified chitosan based coating containing nanoemulsion of essential oils, modified atmosphere packaging and gamma irradiation against Escherichia coli O157:H7 and Salmonella typhimurium on green beans. Food Control 2015, 50, 215–222. [Google Scholar] [CrossRef]
- Goñi, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- López, P.; Sánchez, C.; Batlle, R.; Nerín, C. Solid- and vapor-phase antimicrobial activities of six essential oils: Susceptibility of selected foodborne bacterial and fungal strains. J. Agric. Food Chem. 2005, 53, 6939–6946. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.V.; Easton, C.D.; Anderson, L.J.; Bazaka, K. RF plasma polymerised thin films from natural resources. Int. J. Mod. Phys. Conf. Ser. 2014, 32, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Grant, D.S.; Bazaka, K.; Jacob, M.V. Tailoring terpenoid plasma polymer properties by controlling the substrate temperature during pecvd. J. Appl. Polym. Sci. 2018, 135, 45771. [Google Scholar] [CrossRef]
- Lopez, G.P.; Ratner, B.D. Substrate temperature effects on film chemistry in plasma deposition of organics. 1. Nonpolymerizable precursors. Langmuir 1991, 7, 766–773. [Google Scholar] [CrossRef]
- Shi, F.F. Recent advances in polymer thin films prepared by plasma polymerization synthesis, structural characterization, properties and applications. Surf. Coat. Technol. 1996, 82, 1–15. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.; Chrzanowski, W.; Ostrikov, K. Anti-bacterial surfaces: Natural agents, mechanisms of action, and plasma surface modification. RSC Adv. 2015, 5, 48739–48759. [Google Scholar] [CrossRef]
- Brady, A.; Loughlin, R.; Gilpin, D.; Kearney, P.; Tunney, M. In vitro activity of tea-tree oil against clinical skin isolates of meticillin-resistant and-sensitive Staphylococcus aureus and coagulase-negative staphylococci growing planktonically and as biofilms. J. Med. Microbiol. 2006, 55, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Mondello, F.; De Bernardis, F.; Girolamo, A.; Salvatore, G.; Cassone, A. In vitro and in vivo activity of tea tree oil against azole-susceptible and-resistant human pathogenic yeasts. J. Antimicrob. Chemother. 2003, 51, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.V.; Truong, V.K.; Crawford, R.J.; Ivanova, E.P. The effect of polyterpenol thin film surfaces on bacterial viability and adhesion. Polymers 2011, 3, 388–404. [Google Scholar] [CrossRef] [Green Version]
- Bazaka, K.; Jacob, M.; Truong, V.; Wang, F.; Pushpamali, W.; Wang, J.; Ellis, A.; Berndt, C.; Crawford, R.; Ivanova, E. Effect of plasma-enhanced chemical vapour deposition on the retention of antibacterial activity of terpinen-4-ol. Biomacromolecules 2010, 11, 2016. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.V.; Ivanova, E.P. A study of a retention of antimicrobial activity by plasma polymerized terpinen-4-ol thin films. In Materials Science Forum; Trans Tech Publ: Zürich, Switzerland, 2010; pp. 2261–2264. [Google Scholar]
- Bayram, O.; Simsek, O. Investigation of the effect of RF energy on optical, morphological, chemical and antibacterial properties of polyterpenol thin films obtained by RF-PECVD technique. J. Mater. Sci. Mater. Electron. 2018, 29, 6586–6593. [Google Scholar] [CrossRef]
- Aggarwal, K.K.; Khanuja, S.P.S.; Ahmad, A.; Santha Kumar, T.R.; Gupta, V.K.; Kumar, S. Antimicrobial activity profiles of the two enantiomers of limonene and carvone isolated from the oils of Mentha spicata and Anethum sowa. Flavour Fragr. J. 2002, 17, 59–63. [Google Scholar] [CrossRef]
- McGeady, P.; Wansley, D.L.; Logan, D.A. Carvone and perillaldehyde interfere with the serum-induced formation of filamentous structures in Candida albicans at substantially lower concentrations than those causing significant inhibition of growth. J. Nat. Prod. 2002, 65, 953–955. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C.C.R.; da Fonseca, M.M.R. Carvone: Why and how should one bother to produce this terpene. Food Chem. 2006, 95, 413–422. [Google Scholar] [CrossRef]
- Chan, Y.W.; Siow, K.S.; Ng, P.Y.; Gires, U.; Majlis, B.Y. Plasma polymerized carvone as an antibacterial and biocompatible coating. Mater. Sci. Eng. C 2016, 68, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Juergens, U.; Dethlefsen, U.; Steinkamp, G.; Gillissen, A.; Repges, R.; Vetter, H. Anti-inflammatory activity of 1,8-cineol (Eucalyptol) in bronchial asthma: A double-blind placebo-controlled trial. Respir. Med. 2003, 97, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Dalleau, S.; Cateau, E.; Bergès, T.; Berjeaud, J.-M.; Imbert, C. In vitro activity of terpenes against Candida biofilms. Int. J. Antimicrob. Agents 2008, 31, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Serafino, A.; Vallebona, P.S.; Andreola, F.; Zonfrillo, M.; Mercuri, L.; Federici, M.; Rasi, G.; Garaci, E.; Pierimarchi, P. Stimulatory effect of eucalyptus essential oil on innate cell-mediated immune response. BMC Immunol. 2008, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juergens, U.R.; Engelen, T.; Racké, K.; Stöber, M.; Gillissen, A.; Vetter, H. Inhibitory activity of 1,8-cineol (eucalyptol) on cytokine production in cultured human lymphocytes and monocytes. Pulm. Pharmacol. Ther. 2004, 17, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Pegalajar-Jurado, A.; Easton, C.D.; Styan, K.E.; McArthur, S.L. Antibacterial activity studies of plasma polymerised cineole films. J. Mater. Chem. B 2014, 2, 4993–5002. [Google Scholar] [CrossRef]
- Mann, M.N.; Fisher, E.R. Investigation of antibacterial 1,8-cineole-derived thin films formed via plasma-enhanced chemical vapor deposition. ACS Appl. Mater. Interfaces 2017, 9, 36548–36560. [Google Scholar] [CrossRef] [PubMed]
- Jalali-Heravi, M.; Zekavat, B.; Sereshti, H. Characterization of essential oil components of iranian geranium oil using gas chromatography–mass spectrometry combined with chemometric resolution techniques. J. Chromatogr. A 2006, 1114, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Jones, V.; Buck, R.; Shawcross, S.G.; Dawson, M.M.; Dunn, K. The effect of essential oils on methicillin-resistant Staphylococcus aureus using a dressing model. Burns 2004, 30, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumaili, A.; Bazaka, K.; Jacob, M.V. Retention of antibacterial activity in geranium plasma polymer thin films. Nanomaterials 2017, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. The electrical properties of plasma-deposited thin films derived from Pelargonium graveolens. Electronics 2017, 6, 86. [Google Scholar] [CrossRef]
- Tegoulia, V.A.; Cooper, S.L. Staphylococcus aureus adhesion to self-assembled monolayers: Effect of surface chemistry and fibrinogen presence. Colloids Surf. B Biointerfaces 2002, 24, 217–228. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V. Nanotribological and nanomechanical properties of plasma-polymerized polyterpenol thin films. J. Mater. Res. 2011, 26, 2952–2961. [Google Scholar] [CrossRef]
- Bae, I.; Jung, C.; Cho, S.; Song, Y.; Boo, J. Characterization of organic polymer thin films deposited using the PECVD method. J. Korean Phys. Soc. 2007, 50, 1854–1857. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V. Effects of iodine doping on optoelectronic and chemical properties of polyterpenol thin films. Nanomaterials 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K.; Griesser, S.S.; Griesser, H.J. Antibacterial surfaces and coatings produced by plasma techniques. Plasma Process. Polym. 2011, 8, 1010–1023. [Google Scholar] [CrossRef]
- Jampala, S.N.; Sarmadi, M.; Somers, E.; Wong, A.; Denes, F. Plasma-enhanced synthesis of bactericidal quaternary ammonium thin layers on stainless steel and cellulose surfaces. Langmuir 2008, 24, 8583–8591. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.; Cooper, I. Biomaterials and Medical Device-Associated Infections; Elsevier: New York, NY, USA, 2014. [Google Scholar]
- Lischer, S.; Körner, E.; Balazs, D.J.; Shen, D.; Wick, P.; Grieder, K.; Haas, D.; Heuberger, M.; Hegemann, D. Antibacterial burst-release from minimal Ag-containing plasma polymer coatings. J. R. Soc. Interface 2011, 8, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Bayram, O. Determination of the optical and chemical properties of aniline doped plasma polymerized cineole thin films synthesized at various RF powers. J. Mater. Sci. Mater. Electron. 2018, 29, 8564–8570. [Google Scholar] [CrossRef]
- Bazaka, K.; Ahmad, J.; Oelgemöller, M.; Uddin, A.; Jacob, M.V. Photostability of plasma polymerized γ-terpinene thin films for encapsulation of OPV. Sci. Rep. 2017, 7, 45599. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M. Synthesis of radio frequency plasma polymerized non-synthetic terpinen-4-ol thin films. Mater. Lett. 2009, 63, 1594–1597. [Google Scholar] [CrossRef]
- Yasuda, H. Plasma Polymerization; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Ahmad, J.; Bazaka, K.; Whittle, J.D.; Michelmore, A.; Jacob, M.V. Structural characterization of γ-terpinene thin films using mass spectroscopy and X-ray photoelectron spectroscopy. Plasma Process. Polym. 2015, 12, 1085–1094. [Google Scholar] [CrossRef]
- Easton, C.; Jacob, M. Optical characterisation of radio frequency plasma polymerised Lavandula angustifolia essential oil thin films. Thin Solid Films 2009, 517, 4402–4407. [Google Scholar] [CrossRef]
- Taguchi, D.; Manaka, T.; Iwamoto, M.; Bazaka, K.; Jacob, M.V. Analyzing hysteresis behavior of capacitance–voltage characteristics of Izo/C60/Pentacene/Au diodes with a hole-transport electron-blocking polyterpenol layer by electric-field-induced optical second-harmonic generation measurement. Chem. Phys. Lett. 2013, 572, 150–153. [Google Scholar] [CrossRef]
- Ahmad, J.; Bazaka, K.; Jacob, M. Optical and surface characterization of radio frequency plasma polymerized 1-isopropyl-4-methyl-1,4-cyclohexadiene thin films. Electronics 2014, 3, 266–281. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Cho, S.; Han, J.; Hong, B.; Kim, Y.; Yang, S.; Boo, J.-H. High-rate deposition of plasma polymerized thin films using PECVD method and characterization of their optical properties. Surf. Coat. Technol. 2003, 169, 595–599. [Google Scholar] [CrossRef]
- Ahmad, J.; Bazaka, K.; Oelgemöller, M.; Jacob, M.V. Wetting, solubility and chemical characteristics of plasma-polymerized 1-isopropyl-4-methyl-1, 4-cyclohexadiene thin films. Coatings 2014, 4, 527–552. [Google Scholar] [CrossRef] [Green Version]
- Jacob, M.; Bazaka, K. Fabrication of Electronic Materials from Australian Essential Oils; Australian Government: Australia; Rural Industries Research and Development Corporation: Research and Development Corporation, Canberra, 2010.
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Plasma-assisted surface modification of organic biopolymers to prevent bacterial attachment. Acta Biomater. 2011, 7, 2015–2028. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Friedman, M.; Kozukue, N.; Harden, L.A. Cinnamaldehyde content in foods determined by gas chromatography−mass spectrometry. J. Agric. Food Chem. 2000, 48, 5702–5709. [Google Scholar] [CrossRef] [PubMed]
- Bäcktorp, C.; Wass, J.T.J.; Panas, I.; Sköld, M.; Börje, A.; Nyman, G. Theoretical investigation of linalool oxidation. J. Phys. Chem. A 2006, 110, 12204–12212. [Google Scholar] [CrossRef] [PubMed]
- Selvaag, E.; Holm, J.Ø.; Thune, P. Allergic contact dermatitis in an aroma therapist with multiple sensitizations to essential oils. Contact Dermat. 1995, 33, 354–355. [Google Scholar] [CrossRef]
- Bleasel, N.; Tate, B.; Rademaker, M. Allergic contact dermatitis following exposure to essential oils. Aust. J. Dermatol. 2002, 43, 211–213. [Google Scholar] [CrossRef]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Schmidt, E.; Geier, J.; Lessmann, H.; Schnuch, A.; Frosch, P. Contact allergy to essential oils: Current patch test results (2000–2008) from the information network of departments of dermatology (IVDK). Contact Dermat. 2010, 63, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.; Grimbaldeston, M.; Gamble, J.; Drew, J.; Finlay-Jones, J.; Hart, P. Tea tree oil reduces the swelling associated with the efferent phase of a contact hypersensitivity response. Inflamm. Res. 2002, 51, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Tambor, K.; Herman, A. Linalool affects the antimicrobial efficacy of essential oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Karbach, J.; Ebenezer, S.; Warnke, P.; Behrens, E.; Al-Nawas, B. Antimicrobial effect of Australian antibacterial essential oils as alternative to common antiseptic solutions against clinically relevant oral pathogens. Clin. Lab. 2015, 61, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Maddocks-Jennings, W. Critical incident: Idiosyncratic allergic reactions to essential oils. Complement. Ther. Nurs. Midwifery 2004, 10, 58–60. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M. Review on the antimicrobial properties of carbon nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Grant, D.; Alancherry, S.; Al-Jumaili, A.; Bazaka, K.; Jacob, M.V. Plasma polymerization: Electronics and biomedical application. In Plasma Science and Technology for Emerging Economies; Springer: Berlin/Heidelberg, Germany, 2017; pp. 593–657. [Google Scholar]
- Hegemann, D.; Hossain, M.M.; Körner, E.; Balazs, D.J. Macroscopic description of plasma polymerization. Plasma Process. Polym. 2007, 4, 229–238. [Google Scholar] [CrossRef]
- De Masi, L.; Siviero, P.; Esposito, C.; Castaldo, D.; Siano, F.; Laratta, B. Assessment of agronomic, chemical and genetic variability in common basil (Ocimum basilicum L.). Eur. Food Res. Technol. 2006, 223, 273–281. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Lucera, A.; Sinigaglia, M.; Corbo, M.R. Combined effects of thymol, carvacrol and temperature on the quality of non conventional poultry patties. Meat Sci. 2009, 83, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J. Pulsed-plasma polymerization. In The Plasma Chemistry of Polymer Surfaces; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 377–456. [Google Scholar]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Jumaili, A.; Kumar, A.; Bazaka, K.; Jacob, M.V. Plant Secondary Metabolite-Derived Polymers: A Potential Approach to Develop Antimicrobial Films. Polymers 2018, 10, 515. https://doi.org/10.3390/polym10050515
Al-Jumaili A, Kumar A, Bazaka K, Jacob MV. Plant Secondary Metabolite-Derived Polymers: A Potential Approach to Develop Antimicrobial Films. Polymers. 2018; 10(5):515. https://doi.org/10.3390/polym10050515
Chicago/Turabian StyleAl-Jumaili, Ahmed, Avishek Kumar, Kateryna Bazaka, and Mohan V. Jacob. 2018. "Plant Secondary Metabolite-Derived Polymers: A Potential Approach to Develop Antimicrobial Films" Polymers 10, no. 5: 515. https://doi.org/10.3390/polym10050515