Potassium Efflux and Cytosol Acidification as Primary Anoxia-Induced Events in Wheat and Rice Seedlings
Abstract
:1. Introduction
2. Results
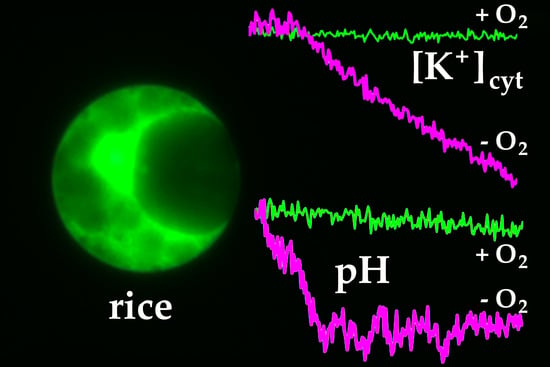
2.1. Influence of Oxygen Deprivation on [K+]cyt in Wheat and Rice Leaf Protoplasts
2.2. Influence of Oxygen Deprivation on pHcyt in Wheat and Rice Leaf Protoplasts
2.3. Influence of Long-Term Anoxia on Potassium Uptake by Intact Wheat and Rice Seedlings
2.4. Influence of Long-Term Anoxia on the pH of the Incubation Medium of Intact Wheat and Rice Seedlings
3. Discussion
3.1. Potassium Changes
3.2. Acidification Caused by Anoxia
3.3. Calcium Involvement in Anoxic Signaling
3.4. A Suggested Model
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Protoplast Isolation and Dye Loading
4.3. Fluorescence Measurements and In Situ Calibration
4.4. In Situ Anoxic Treatment
4.5. Measurement of Potassium Uptake by Roots of Intact Seedlings and pH of Incubation Medium
4.6. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geisler, M.; Venema, K. Transporters and Pumps in Plant Signaling Editors. Signaling and Communication in Plants (Book 7); Springer: Berlin/Heidelberg, Germany, 2011; 388p. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Reddy, P.S.; Ferrante, A.; Khan, N.A. Plant Signaling Molecule: Role and Regulation under Stressful Environments; Elsevier (Woodhead Publishing): Cambridge, UK, 2019; 596p. [Google Scholar] [CrossRef]
- Bose, J.; Pottosin, I.I.; Shabala, S.S.; Palmgren, M.G.; Shabala, S. Calcium efflux systems in stress signaling and adaptation in plants. Front. Plant Sci. 2011, 2, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edel, K.H.; Marchadier, E.; Brownlee, C.; Kudla, J.; Hetherington, A.M. The evolution of calcium-based signalling in plants. Curr. Biol. 2017, 27, 667–679. [Google Scholar] [CrossRef]
- Demidchik, V.; Shabala, S. Mechanisms of cytosolic calcium elevation in plants: The role of ion channels, calcium extrusion systems and NADPH oxidase mediated ‘ROS-Ca2+ Hub’. Funct. Plant Biol. 2018, 45, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Shabala, S.; Isayenkov, S.; Cuin, T.A.; Pottosin, I. Calcium transport across plant membranes: Mechanisms and functions. New Phytol. 2018, 220, 49–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathy, B.C.; Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Waszczak, C.; Carmody, M.; Kangasjarvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [Green Version]
- Talaat, N.B. Role of reactive oxygen species signaling in plant growth and development. Chapter 10. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: New York, NY, USA, 2019; pp. 225–266. [Google Scholar] [CrossRef]
- Choi, W.G.; Miller, G.; Wallace, I.; Harper, J.; Mittler, R.; Gilroy, S. Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant J. 2017, 90, 698–707. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Li, R.L.; Zhang, L.; Wang, Q.L.; Niehaus, K.; Baluška, F.; Šamaj, J.; Lin, J.X. Lipid microdomain polarization is required for NADPH oxidase dependent ROS signaling in Picea meyeri pollen tube tip growth. Plant J. 2009, 60, 303–313. [Google Scholar] [CrossRef]
- Romani, G.; Bonza, M.C.; Filippini, I.; Cerana, M.; Beffagna, N.; De Michelis, M.I. Involvement of the plasma membrane Ca2+-ATPase in the short-term response of Arabidopsis thaliana cultured cells to oligogalacturonides. Plant. Biol. 2004, 6, 192–200. [Google Scholar] [CrossRef]
- Felle, H.H. pH: Signal and messenger in plant cells. Plant Biol. 2001, 3, 577–591. [Google Scholar] [CrossRef]
- Behera, S.; Xu, Z.; Luoni, L.; Bonza, M.C.; Doccula, F.G.; De Michelis, M.I.; Morris, R.J.; Schwarzländer, M.; Costa, A. Cellular Ca2+ signals generate defined pH signatures in plants. Plant Cell 2018, 30, 2704–2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]
- Shabala, S. Signalling by potassium: Another second messenger to add to the list? J. Exp. Bot. 2017, 68, 4003–4007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio, F.; Nieves-Cordones, M.; Horie, T.; Shabala, S. Doing ‘business as usual’ comes with a cost: Evaluating energy cost of maintaining plant intracellular K+ homeostasis under saline conditions. New Phytol. 2020, 225, 1097–1104. [Google Scholar] [CrossRef] [Green Version]
- Gajdanowicz, P.; Michard, E.; Sandmann, M.; Rocha, M.; Corrêa, L.G.G.; Ramírez-Aguilar, S.J.; Gomez-Porras, J.L.; González, W.; Thibaud, J.-B.; van Dongen, J.T.; et al. Potassium (K+) gradients serve as a mobile energy source in plant vascular tissues. Proc. Natl. Acad. Sci. USA 2011, 108, 864–869. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, I.; Gomez-Porras, J.L.; Riedelsberger, J. The potassium battery: A mobile energy source for transport processes in plant vascular tissues. New Phytol. 2017, 216, 1049–1053. [Google Scholar] [CrossRef] [Green Version]
- Shabala, S.; Demidchik, V.; Shabala, L.; Cuin, T.A.; Smith, S.J.; Miller, A.J.; Davies, J.M.; Newman, I.A. Extracellular Ca2+ ameliorates NaCl induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 2006, 141, 1653–1665. [Google Scholar] [CrossRef] [Green Version]
- Rubio, F.; Fon, M.; Rodenas, R.; Nieves-Cordones, M.; Aleman, F.; Rivero, R.M.; Martinez, V. A low K+ signal is required for functional high-affinity K+ uptake through HAK5 transporters. Physiol. Plant. 2014, 152, 558–570. [Google Scholar] [CrossRef]
- Shabala, L.; Zhang, J.; Pottosin, I.; Bose, J.; Zhu, M.; Fuglsang, A.T.; Velarde-Buendia, A.; Massart, A.; Hill, C.B.; Roessner, U.; et al. Cell-type-specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiol. 2016, 172, 2445–2458. [Google Scholar] [CrossRef] [Green Version]
- Eisenach, C.; Papanatsiou, M.; Hillert, E.-K.; Blatt, M.R. Clustering of the K+ channel GORK of Arabidopsis parallels its gating by extracellular K+. Plant J. 2014, 78, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Adem, G.D.; Chen, G.; Shabala, L.; Chen, Z.-H.; Shabala, S. GORK channel: A master switch of plant metabolism? Trends Plant Sci. 2020, 25, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, F.; Thomson, C.J.; Steigner, W.; Barrett-Lennard, E.G.; Gibbs, J.; Greenway, H. Hypoxia induces membrane depolarization and potassium-loss from wheat roots but does not increase their permeability to sorbitol. J. Exp. Bot. 1988, 39, 1169–1183. [Google Scholar] [CrossRef]
- Colmer, T.D.; Huang, S.; Greenway, H. Evidence for down-regulation of ethanolic fermentation and K+ effluxes in the coleoptile of rice seedlings during prolonged anoxia. J. Exp. Bot. 2001, 52, 1507–1517. [Google Scholar] [CrossRef]
- Huang, S.; Ishizawa, K.; Greenway, H.; Colmer, T.D. Manipulation of ethanol production in anoxic rice coleoptiles by exogenous glucose determines rates of ion fluxes and provides estimates of energy requirements for cell maintenance during anoxia. J. Exp. Bot. 2005, 56, 2453–2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, S.; Marras, A.M. Adaptative response of Vitis root to anoxia. Plant Cell Physiol. 2006, 47, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Zeng, F.; Konnerup, D.; Shabala, L.; Zhou, M.; Colmer, T.D.; Zhang, G.; Shabala, S. Linking oxygen availability with membrane potential maintenance and K+ retention of barley roots: Implications for waterlogging stress tolerance. Plant Cell Environ. 2014, 37, 2325–2338. [Google Scholar] [CrossRef]
- Loreti, E.; Perata, P. The many facets of hypoxia in plants. Plants 2020, 9, 745. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.-H.; Shabala, S. Hypoxia sensing in plants: On a quest for ion channels as putative oxygen sensors. Plant Cell Physiol. 2017, 58, 1126–1142. [Google Scholar] [CrossRef]
- Shabala, S.; Shabala, L.; Barcelo, J.; Poschenrieder, C. Membrane transporters mediating root signalling and adaptive responses to oxygen deprivation and soil flooding. Plant Cell Environ. 2014, 37, 2216–2233. [Google Scholar] [CrossRef]
- Subbaiah, C.C.; Sachs, M.M. Molecular and cellular adaptations of maize to flooding stress. Ann. Bot. 2003, 91, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Yemelyanov, V.V.; Shishova, M.F.; Chirkova, T.V.; Lindberg, S.M. Anoxia-induced elevation of cytosolic Ca2+ concentration depends on different Ca2+ sources in rice and wheat protoplasts. Planta 2011, 234, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, S.; Kader, M.A.; Yemelyanov, V. Calcium signalling in plant cells under environmental stress. In Plant Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA; Dordrecht, The Netherlands; Heidelberg, Germany; London, UK, 2012; pp. 325–360. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Hill, R.D. Elevation of cytosolic Ca2+ in response to energy deficiency in plants: The general mechanism of adaptation to low oxygen stress. Biochem. J. 2018, 475, 1411–1425. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, Z.-H.; Liu, X.; Colmer, T.D.; Shabala, L.; Salih, A.; Zhou, M.; Shabala, S. Revealing the roles of GORK channels and NADPH oxidase in acclimation to hypoxia in Arabidopsis. J. Exp. Bot. 2017, 68, 3191–3204. [Google Scholar] [CrossRef] [Green Version]
- Pang, J.Y.; Newman, I.; Mendham, N.; Zhou, M.; Shabala, S. Microelectrode ion and O2 fluxes measurements reveal differential sensitivity of barley root tissues to hypoxia. Plant Cell Environ. 2006, 29, 1107–1121. [Google Scholar] [CrossRef]
- He, L.; Li, B.; Lu, X.; Yuan, L.; Yang, Y.; Yuan, Y.; Du, J.; Guo, S. The effect of exogenous calcium on mitochondria, respiratory metabolism enzymes and ion transport in cucumber roots under hypoxia. Sci. Rep. 2015, 5, 11391. [Google Scholar] [CrossRef] [Green Version]
- Felle, H.H. pH regulation in anoxic plants. Ann. Bot. 2005, 96, 519–532. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.K.M.; Callis, J.; Jardetzky, O.; Walbot, V.; Freeling, M. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proc. Natl. Acad. Sci. USA 1984, 81, 6029–6033. [Google Scholar] [CrossRef] [Green Version]
- Saint-Ges, V.; Roby, C.; Bligny, R.; Pradet, A.; Douce, R. Kinetic studies of the variation of cytoplasmic pH, nucleotide triphosphates (31P-NMR) and lactate during normoxic and anoxic transitions in maize root tips. Eur. J. Biochem. 1991, 200, 477–482. [Google Scholar] [CrossRef]
- Fox, G.G.; McCallan, N.R.; Ratcliffe, R.G. Manipulating cytoplasmic pH under anoxia: A critical test of the role of pH in the switch from aerobic to anaerobic metabolism. Planta 1995, 195, 324–330. [Google Scholar] [CrossRef]
- Gout, E.; Boisson, A.-M.; Aubert, S.; Douce, R.; Bligny, R. Origin of the cytoplasmic pH changes during anaerobic stress in higher plant cells. Carbon-13 and Phosphorous-31 nuclear magnetic resonance studies. Plant Physiol. 2001, 125, 912–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menegus, F.; Cattaruzza, L.; Mattana, M.; Beffagna, N.; Ragg, E. Response to anoxia in rice and wheat seedlings changes in the pH of intracellular compartments, glucose-6-phosphate level, and metabolic rate. Plant Physiol. 1991, 95, 760–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulichikhin, K.Y.; Aitio, O.; Chirkova, T.V.; Fagerstedt, K.V. Effect of oxygen concentration on intracellular pH, glucose-6-phosphate and NTP content in rice (Oryza sativa) and wheat (Triticum aestivum) root tips: In vivo 31P-NMR study. Physiol. Plant. 2007, 129, 507–518. [Google Scholar] [CrossRef]
- Greenway, H.; Gibbs, J. Mechanisms of anoxia tolerance in plants. II. Energy requirement for maintenance and energy distribution to essential processes. Funct. Plant Biol. 2003, 30, 999–1036. [Google Scholar] [CrossRef]
- Drew, M.C. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Sakano, K.; Kiyota, S.; Yasaki, Y. Acidification and alkalization of culture medium by Carathanus roseus cells—Is anoxic production of lactate a cause of cytoplasmic acidification? Plant Cell Physiol. 1997, 38, 1053–1059. [Google Scholar] [CrossRef] [Green Version]
- Chirkova, T.; Yemelyanov, V. The study of plant adaptation to oxygen deficiency in Saint Petersburg University. Biol. Commun. 2018, 63, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Subbaiah, C.; Bush, D.S.; Sachs, M. Elevation of cytosolic calcium precedes anoxic gene expression in maize suspension cultured cells. Plant Cell 1994, 6, 1747–1762. [Google Scholar] [CrossRef] [Green Version]
- Sedbrook, J.C.; Kronebusch, P.J.; Borisy, G.G.; Trewavas, A.J.; Masson, P.H. Transgenic AEQUORIN reveals organ-specific cytosolic Ca2+ responses to anoxia in Arabidopsis thaliana seedlings. Plant Physiol. 1996, 111, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Biemelt, S.; Keetman, U.; Albrecht, G. Re-aeration following hypoxia or anoxia leads to activation of the antioxidative defense system in roots of wheat seedlings. Plant Physiol. 1998, 116, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Edwards, G.E.S.P.; Robinson, S.P.; Tyler, N.J.E.; Walker, D.A. Photosynthesis by isolated protoplasts, protoplast extracts and chloroplasts of wheat. Plant Physiol. 1978, 62, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindberg, S.; Strid, H. Aluminium induces rapid changes in cytosolic pH and free calcium and potassium concentrations in root protoplasts of wheat (Triticum aestivum). Physiol. Plant. 1997, 99, 405–414. [Google Scholar] [CrossRef]
- Shishova, M.; Lindberg, S. Auxin-Induced cytosolic acidification in wheat leaf protoplasts depends on external concentration of Ca2+. J. Plant Physiol. 1999, 155, 190–196. [Google Scholar] [CrossRef]
- Lindberg, S. In-situ determination of intracellular concentrations of K+ in barley (Hordeum vulgare L. cv. Kara) using the K+-binding fluorescent dye benzofuran isophthalate. Planta 1995, 195, 525–529. [Google Scholar] [CrossRef]
- Morgan, S.H.; Maity, P.J.; Geilfus, C.-M.; Lindberg, S.; Mühling, K.H. Leaf ion homeostasis and plasma membrane H+-ATPase activity in Vicia faba change after extra calcium and potassium supply under salinity. Plant Physiol. Biochem. 2014, 82, 244–253. [Google Scholar] [CrossRef]
- Phillips, H.J. Dye expulsion tests for cell viability. In Tissue Cultures: Methods and Application. Chapter 3, Section VIII—Evaluation of Culture Dynamics; Kruse, P.F., Patterson, M.K., Eds.; Academic Press: New York, NY, USA, 1973; p. 406. [Google Scholar]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yemelyanov, V.V.; Chirkova, T.V.; Shishova, M.F.; Lindberg, S.M. Potassium Efflux and Cytosol Acidification as Primary Anoxia-Induced Events in Wheat and Rice Seedlings. Plants 2020, 9, 1216. https://doi.org/10.3390/plants9091216
Yemelyanov VV, Chirkova TV, Shishova MF, Lindberg SM. Potassium Efflux and Cytosol Acidification as Primary Anoxia-Induced Events in Wheat and Rice Seedlings. Plants. 2020; 9(9):1216. https://doi.org/10.3390/plants9091216
Chicago/Turabian StyleYemelyanov, Vladislav V., Tamara V. Chirkova, Maria F. Shishova, and Sylvia M. Lindberg. 2020. "Potassium Efflux and Cytosol Acidification as Primary Anoxia-Induced Events in Wheat and Rice Seedlings" Plants 9, no. 9: 1216. https://doi.org/10.3390/plants9091216