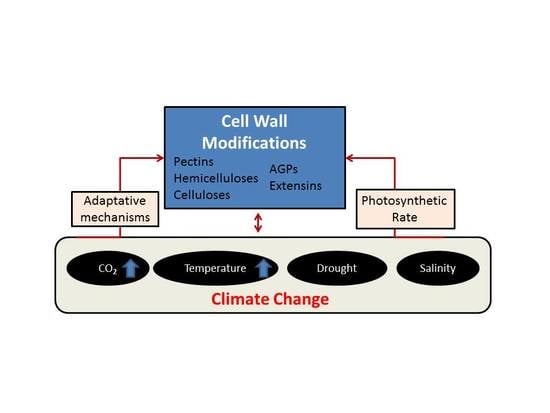
Plant Cell Walls Tackling Climate Change: Biotechnological Strategies to Improve Crop Adaptations and Photosynthesis in Response to Global Warming
Abstract
:1. Introduction
1.1. Plants Are Necessary to Remove CO2 from the Atmosphere
1.2. Impact of Climate Change in Plants: Stress Responses and Adaptation
2. Discussion
2.1. Plant Cell Wall Modifications Are Coordinated to Developmental Processes
2.1.1. Following the Cycle of Carbon in Plants: From Carbon Fixation to Cell Wall Formation
2.1.2. Cell Wall Proteins Play a Key Role in Developmental Processes
2.1.3. The Interaction of Cell Wall Polymers in Developmental Processes
2.2. The Impact of Climate Change-Induced High Photosynthesis in Cell Wall
Cell Wall Homeostasis Responds to Alterations in Photosynthesis
2.3. Cell Wall Remodeling under Salinity Stress
Cell Wall Proteins Are Active Players in Response to Salinity
2.4. The Effect of Elevated CO2 Concentrations and Cell Wall Modifications
2.4.1. Cell Wall Mechanical Properties and Structure Is Modulated in Response to High CO2
2.4.2. Cell Wall Biosynthesis and Biomass Is Increased in Response to Elevated CO2
2.5. Cell Wall Remodeling under Drought Stress
2.6. The Effect of Elevated Temperatures in Cell Wall Reorganization
2.6.1. Cell Wall Composition Is Altered in Response to High Temperature
2.6.2. AGPs Respond to High Temperature Stress
3. Conclusions
4. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CW | cell wall |
| Suc | sucrose |
| Glu | glucose |
| Fru | fructose |
| Fru-6-P | fructose-6-phosphate |
| CesA | cellulose synthase |
| CW INV | cell-wall invertase |
| INV | invertase |
| SuSy | sucrose synthase |
| WT | wildtype |
| PME | pectin methylesterase |
| PMEI | pectin methylestersase inhibitor |
| CSL | CesA-like protein |
| PAE | pectin acetylesterase |
| GPRs | glycine-rich proteins |
| AGPs | Arabinogalactan proteins |
| EXL | expansin-like |
| XTH | xyloglucan endotransglucosylase |
| FLA | fasciclin-like arabinogalactan-proteins |
| FER | feronia |
| HG | homogalacturonan |
| RLK | receptors-like kinase |
| XET | xyloglucan endotransglycosylase |
| EXP | expansin |
| GT | galacturonosyltransferase-like |
| BIN | brassinosteroid insensitive |
| BR | Brassinosteroid |
| GH | glycoside hydrolases |
| UGPase | UDP-glucose pyrophosphorylase |
| CO2 | carbon dioxide |
| C | carbon |
| UDP | uridine diphosphate glucose |
| HGRPs | hydroxyproline-rich glycoproteins |
| GPI | glycosylphosphatidylinositol |
| HSPs | heat shock proteins |
| RGI | rhamnogalacturonan I |
| RGII | rhamnogalacturonan II |
| XET | xyloglucan endotransglucosylase |
| MUR4 | UDP-D-xylose 4-epimerase |
| SOS | salt overly sensitive |
References
- Ripple, W.J.; Wolf, C.; Newsome, T.M.; Barnard, P.; Moomaw, W.R. World Scientists’ Warning of a Climate Emergency. Bioscience 2019, 2019, 8–12. [Google Scholar] [CrossRef]
- Tong, S.; Ebi, K. Preventing and mitigating health risks of climate change. Environ. Res. 2019, 174, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parain, E.C.; Rohr, R.P.; Gray, S.M.; Bersier, L.F. Increased temperature disrupts the biodiversity–Ecosystem functioning relationship. Am. Nat. 2019, 193, 227–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhenmin, L.; Espinosa, P. Tackling climate change to accelerate sustainable development. Nat. Clim. Chang. 2019, 9, 494–496. [Google Scholar] [CrossRef]
- Bain, P.G.; Bongiorno, R. It’s not too late to do the right thing: Moral motivations for climate change action. Wiley Interdiscip. Rev. Clim. Chang. 2020, 11, e615. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.O.H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Summary for Policymakers. In Global Warming of 1.5°C; An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Griscom, B.W.; Adams, J.; Ellis, P.W.; Houghton, R.A.; Lomax, G.; Miteva, D.A.; Schlesinger, W.H.; Shoch, D.; Siikamäki, J.V.; Smith, P.; et al. Natural climate solutions. Proc. Natl. Acad. Sci. USA 2017, 114, 11645–11650. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Bonan, G.B.; Doney, S.C. Climate, ecosystems, and planetary futures: The challenge to predict life in Earth system models. Science 2018, 359, eaam8328. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Liu, H.; Hua, L.; Luo, Q.; Lin, Y.; He, P.; Feng, S.; Liu, J.; Ye, Q. Differential Responses of Stomata and Photosynthesis to Elevated Temperature in Two Co-occurring Subtropical Forest Tree Species. Front. Plant Sci. 2018, 9, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamann, T. The plant cell wall integrity maintenance mechanism \textendash A case study of a cell wall plasma membrane signaling network. Phytochemistry 2015, 112, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Hamann, T. Plant cell wall integrity maintenance as an essential component of biotic stress response mechanisms. Front. Plant Sci. 2012, 3, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, M.R.; Koltunow, A.M.G. Traffic monitors at the cell periphery: The role of cell walls during early female reproductive cell differentiation in plants. Curr. Opin. Plant Biol. 2014, 17, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Voiniciuc, C.; Fu, L.; Dieluweit, S.; Klose, H.; Usadel, B. TRM4 is essential for cellulose deposition in Arabidopsis seed mucilage by maintaining cortical microtubule organization and interacting with CESA3. New Phytol. 2019, 221, 881–895. [Google Scholar] [CrossRef]
- Burn, J.E.; Hurley, U.A.; Birch, R.J.; Arioli, T.; Cork, A.; Williamson, R.E. The cellulose-deficient Arabidopsis mutant rsw3 is defective in a gene encoding a putative glucosidase II, an enzyme processing N-glycans during ER quality control. Plant J. 2002, 32, 949–960. [Google Scholar] [CrossRef]
- Beeckman, T.; Przemeck, G.K.H.; Stamatiou, G.; Lau, R.; Terryn, N.; De Rycke, R.; Inzé, D.; Berleth, T. Genetic complexity of cellulose synthase A gene function in arabidopsis embryogenesis. Plant Physiol. 2002, 130, 1883–1893. [Google Scholar] [CrossRef] [Green Version]
- Sampathkumar, A.; Peaucelle, A.; Fujita, M.; Schuster, C.; Persson, S.; Wasteneys, G.O.; Meyerowitz, E.M. Primary wall cellulose synthase regulates shoot apical meristem mechanics and growth. Development 2019, 146, dev179036. [Google Scholar] [CrossRef] [Green Version]
- Talbott, L.D.; Ray, P.M. Molecular size and separability features of pea cell wall polysaccharides: Implications for models of primary wall structure. Plant Physiol. 1992, 98, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Shigeyama, T.; Watanabe, A.; Tokuchi, K.; Toh, S.; Sakurai, N.; Shibuya, N.; Kawakami, N. α-Xylosidase plays essential roles in xyloglucan remodelling, maintenance of cell wall integrity, and seed germination in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 5615–5629. [Google Scholar] [CrossRef] [Green Version]
- Günl, M.; Pauly, M. AXY3 encodes a α-xylosidase that impacts the structure and accessibility of the hemicellulose xyloglucan in Arabidopsis plant cell walls. Planta 2011, 233, 707–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampedro, J.; Pardo, B.; Gianzo, C.; Guitián, E.; Revilla, G.; Zarra, I. Lack of α-xylosidase activity in arabidopsis alters xyloglucan composition and results in growth defects. Plant Physiol. 2010, 154, 1105–1115. [Google Scholar] [CrossRef] [Green Version]
- Willats, W.G.; Orfila, C.; Limberg, G.; Buchholt, H.C.; van Alebeek, G.J.; Voragen, A.G.; Marcus, S.E.; Christensen, T.M.; Mikkelsen, J.D.; Murray, B.S.; et al. Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. Implications for pectin methyl esterase action, matrix properties, and cell adhesion. J. Biol. Chem. 2001, 276, 19404–19413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelloux, J.; Rustérucci, C.; Mellerowicz, E.J. New Insights into Pectin Methylesterase Structure and Function. Trends Plant Sci. 2007, 12, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Ryden, P.; Sugimoto-Shirasu, K.; Smith, A.C.; Findlay, K.; Reiter, W.D.; McCann, M.C. Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiol. 2003, 132, 1033–1040. [Google Scholar] [CrossRef] [Green Version]
- Baluška, F.; Liners, F.; Hlavačka, A.; Schlicht, M.; Van Cutsem, P.; McCurdy, D.W.; Menzel, D. Cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate within cell plates during cytokinesis. Protoplasma 2005, 225, 141–155. [Google Scholar] [CrossRef]
- Bárány, I.; Fadón, B.; Risueño, M.C.; Testillano, P.S. Cell wall components and pectin esterification levels as markers of proliferation and differentiation events during pollen development and pollen embryogenesis in Capsicum annuum L. J. Exp. Bot. 2010, 61, 1159–1175. [Google Scholar] [CrossRef]
- Muller, K.; Levesque-Tremblay, G.; Bartels, S.; Weitbrecht, K.; Wormit, A.; Usadel, B.; Haughn, G.; Kermode, A.R. Demethylesterification of Cell Wall Pectins in Arabidopsis Plays a Role in Seed Germination. Plant Physiol. 2013, 161, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Ezquer, I.; Mizzotti, C.; Nguema-Ona, E.; Gotté, M.; Beauzamy, L.; Viana, V.E.; Dubrulle, N.; Costa de Oliveira, A.; Caporali, E.; Koroney, A.-S.; et al. The Developmental Regulator SEEDSTICK Controls Structural and Mechanical Properties of the Arabidopsis Seed Coat. Plant Cell 2016, 28, 2478–2492. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, Y.; Qu, M.; Xiao, H.; Feng, Y.; Liu, J.; Wu, L.; Yu, M. Cell wall pectin and its methyl-esterification in transition zone determine Al resistance in cultivars of pea (Pisum sativum). Front. Plant Sci. 2016, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Geng, X.; Horst, W.J.; Golz, J.F.; Lee, J.E.; Ding, Z.; Yang, Z.B. LEUNIG_HOMOLOG transcriptional co-repressor mediates aluminium sensitivity through PECTIN METHYLESTERASE46-modulated root cell wall pectin methylesterification in Arabidopsis. Plant J. 2017, 90, 491–504. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Ubaldo, H.; de Folter, S. Exploring Cell Wall Composition and Modifications during the Development of the Gynoecium Medial Domain in Arabidopsis. Front. Plant Sci. 2018, 9, 454. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Ubaldo, H.; Lozano-Sotomayor, P.; Ezquer, I.; Di Marzo, M.; Chávez Montes, R.A.; Gómez-Felipe, A.; Pablo-Villa, J.; Diaz-Ramirez, D.; Ballester, P.; Ferrándiz, C.; et al. New roles of NO TRANSMITTING TRACT and SEEDSTICK during medial domain development in Arabidopsis fruits. Development 2019, 146, dev172395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decker, D.; Kleczkowski, L.A. UDP-sugar producing pyrophosphorylases: Distinct and essential enzymes with overlapping substrate specificities, providing de novo precursors for glycosylation reactions. Front. Plant Sci. 2019, 9, 1822. [Google Scholar] [CrossRef] [PubMed]
- Verbančič, J.; Lunn, J.E.; Stitt, M.; Persson, S. Carbon Supply and the Regulation of Cell Wall Synthesis. Mol. Plant 2018, 11, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G. Fascinating Fasciclins: A Surprisingly Widespread Family of Proteins that Mediate Interactions between the Cell Exterior and the Cell Surface. Int. J. Mol. Sci. 2018, 19, 1628. [Google Scholar] [CrossRef] [Green Version]
- Stein, O.; Granot, D. An Overview of Sucrose Synthases in Plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Krasensky, J.; Broyart, C.; Rabanal, F.A.; Jonak, C. The Redox-Sensitive Chloroplast Trehalose-6-Phosphate Phosphatase AtTPPD Regulates Salt Stress Tolerance. Antioxid. Redox Signal. 2014, 21, 1289–1304. [Google Scholar] [CrossRef] [Green Version]
- Ebrecht, A.C.; Asención Diez, M.D.; Piattoni, C.V.; Guerrero, S.A.; Iglesias, A.A. The UDP-glucose pyrophosphorylase from Giardia lamblia is redox regulated and exhibits promiscuity to use galactose-1-phosphate. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 88–96. [Google Scholar] [CrossRef]
- Persia, D.; Cai, G.; Del Casino, C.; Faleri, C.; Willemse, M.T.M.; Cresti, M. Sucrose Synthase Is Associated with the Cell Wall of Tobacco Pollen Tubes. Plant Physiol. 2008, 147, 1603–1618. [Google Scholar] [CrossRef] [Green Version]
- Baroja-Fernández, E.; Muñoz, F.J.; Saikusa, T.; Rodríguez-López, M.; Akazawa, T.; Pozueta-Romero, J. Sucrose synthase catalyzes the de novo production of ADPglucose linked to starch biosynthesis in heterotrophic tissues of plants. Plant Cell Physiol. 2003, 44, 500–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amor, Y.; Haigler, C.H.; Johnson, S.; Wainscott, M.; Delmer, D.P. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc. Natl. Acad. Sci. USA 1995, 92, 9353–9357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, D. Examining the Roles of Sucrose Synthase Isoforms in Arabidopsis Growth and Development. Ph.D. Thesis, University of British Columbia, University of British Columbia, Vancouver, BC, Canada, 2019. [Google Scholar] [CrossRef]
- Bahaji, A.; Li, J.; Sánchez-López, A.; Baroja-Fernández, E.; Muñoz, F.J.F.; Sánchez-López, Á.M.; Baroja-Fernández, E.; Muñoz, F.J.F.; Ovecka, M.; Almagro, G.; et al. Starch biosynthesis, its regulation and biotechnological approaches to improve crop yields. Biotechnol. Adv. 2014, 32, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Ezquer, I.; Li, J.; Ovecka, M.; Baroja-Fernández, E.; Muñoz, F.J.; Montero, M.; de Cerio, J.D.; Hidalgo, M.; Sesma, M.T.; Bahaji, A.; et al. A suggested model for potato MIVOISAP involving functions of central carbohydrate and amino acid metabolism, as well as actin cytoskeleton and endocytosis. Plant Signal. Behav. 2010, 5, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Xu, S.; Zhang, Z.; Chen, G.; Zhong, Y.; Liu, L.; Zhang, R.; Xue, J.; Guo, D. Evolutionary, structural and expression analysis of core genes involved in starch synthesis. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Preiss, J. Plant Starch Synthesis. In Starch in Food: Structure, Function and Applications, 2nd ed.; Elsevier Inc.: Philadelphia, PA, USA, 2018; pp. 3–95. ISBN 9780081008966. [Google Scholar]
- Fragkostefanakis, S.; Sedeek, K.E.M.; Raad, M.; Zaki, M.S.; Kalaitzis, P. Virus induced gene silencing of three putative prolyl 4-hydroxylases enhances plant growth in tomato (Solanum lycopersicum). Plant Mol. Biol. 2014, 85, 459–471. [Google Scholar] [CrossRef]
- Sommer-Knudsen, J.; Bacic, A.; Clarke, A.E. Hydroxyproline-rich plant glycoproteins. Phytochemistry 1998, 47, 483–497. [Google Scholar] [CrossRef]
- Leach, J.E.; Cantrell, M.A.; Sequeira, L. Hydroxyproline-Rich Bacterial Agglutinin from Potato. Plant Physiol. 1982, 70, 1353–1358. [Google Scholar] [CrossRef] [Green Version]
- Esquerré-tugayé, M.T.; Mazau, D. Effect of a fungal disease on extensin, the plant cell wall glycoprotein. J. Exp. Bot. 1974, 25, 509–513. [Google Scholar] [CrossRef]
- Cassab, G.I.; Varner, J.E. Cell Wall Proteins. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 321–353. [Google Scholar] [CrossRef]
- Li, S.X.; Showalter, A.M. Cloning and developmental/stress-regulated expression of a gene encoding a tomato arabinogalactan protein. Plant Mol. Biol. 1996, 32, 641–652. [Google Scholar] [CrossRef]
- Ellis, M.; Egelund, J.; Schultz, C.J.; Bacic, A. Arabinogalactan-Proteins: Key Regulators at the Cell Surface? Plant Physiol. 2010, 153, 403–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Showalter, A.M.; Basu, D. Extensin and Arabinogalactan-Protein Biosynthesis: Glycosyltransferases, Research Challenges, and Biosensors. Front. Plant Sci. 2016, 7, 814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borner, G.H.H.; Lilley, K.S.; Stevens, T.J.; Dupree, P. Identification of Glycosylphosphatidylinositol-Anchored Proteins in Arabidopsis. A Proteomic and Genomic Analysis. Plant Physiol. 2003, 132, 568–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fincher, G.B.; Stone, B.A.; Clarke, A.E. Arabinogalactan-Proteins: Structure, Biosynthesis, and Function. Annu. Rev. Plant Physiol. 1983, 34, 47–70. [Google Scholar] [CrossRef]
- Nothnagel, E.A. Proteoglycans and Related Components in Plant Cells. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 1997; pp. 195–291. [Google Scholar]
- Lu, H.; Chen, M.; Showalter, A.M. Developmental expression and perturbation of arabinogalactan-proteins during seed germination and seedling growth in tomato. Physiol. Plant. 2001, 112, 442–450. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Knox, J.P. A role for arabinogalactan-proteins in plant cell expansion: Evidence from studies on the interaction of beta-glucosyl Yariv reagent with seedlings of Arabidopsis thaliana. Plant J. 1996, 9, 919–925. [Google Scholar] [CrossRef]
- Yang, J.; Sardar, H.S.; McGovern, K.R.; Zhang, Y.; Showalter, A.M. A lysine-rich arabinogalactan protein in Arabidopsis is essential for plant growth and development, including cell division and expansion. Plant J. 2007, 49, 629–640. [Google Scholar] [CrossRef]
- Serpe, M.D.; Nothnagel, E.A. Heterogeneity of arabinogalactan-proteins on the plasma membrane of rose cells. Plant Physiol. 1996, 112, 1261–1271. [Google Scholar] [CrossRef] [Green Version]
- Langan, K.J.; Nothnagel, E.A. Cell surface arabinogalactan-proteins and their relation to cell proliferation and viability. Protoplasma 1997, 196, 87–98. [Google Scholar] [CrossRef]
- Guan, Y.; Nothnagel, E.A. Binding of Arabinogalactan Proteins by Yariv Phenylglycoside Triggers Wound-Like Responses in Arabidopsis Cell Cultures. Plant Physiol. 2004, 135, 1346–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fragkostefanakis, S.; Dandachi, F.; Kalaitzis, P. Expression of arabinogalactan proteins during tomato fruit ripening and in response to mechanical wounding, hypoxia and anoxia. Plant Physiol. Biochem. 2012, 52, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Showalter, A.M. Yariv reagent treatment induces programmed cell death in Arabidopsis cell cultures and implicates arabinogalactan protein involvement. Plant J. 1999, 19, 321–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamport, D.T.A.; Kieliszewski, M.J.; Showalter, A.M. Salt stress upregulates periplasmic arabinogalactan proteins: Using salt stress to analyse AGP function*. New Phytol. 2006, 169, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Fragkostefanakis, S.; Kaloudas, D.; Kalaitzis, P. Pyridine 2, 4-Dicarboxylic Acid Suppresses Tomato Seedling Growth. Front. Chem. 2018, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Baldoni, E.; Genga, A.; Cominelli, E. Plant MYB transcription factors: Their role in drought response mechanisms. Int. J. Mol. Sci. 2015, 16, 15811–15851. [Google Scholar] [CrossRef] [Green Version]
- Phukan, U.J.; Jeena, G.S.; Shukla, R.K. WRKY Transcription Factors: Molecular Regulation and Stress Responses in Plants; Frontiers Media S.A.: Lausanne, Switzerland, 2016. [Google Scholar]
- Viana, V.E.; Marini, N.; Finatto, T.; Ezquer, I.; Busanello, C.; dos Santos, R.S.; Pegoraro, C.; Colombo, L.; Costa de Oliveira, A. Iron excess in rice: From phenotypic changes to functional genomics of WRKY transcription factors. Genet. Mol. Res. 2017, 16, 1–16. [Google Scholar] [CrossRef]
- Castelán-Muñoz, N.; Herrera, J.; Cajero-Sánchez, W.; Arrizubieta, M.; Trejo, C.; García-Ponce, B.; Sánchez, M.; de la Paz, S.; Álvarez-Buylla, E.R.; Garay-Arroyo, A. MADS-box genes are key components of genetic regulatory networks involved in abiotic stress and plastic developmental responses in plants. Front. Plant Sci. 2019, 10, 853. [Google Scholar] [CrossRef] [Green Version]
- Klay, I.; Gouia, S.; Liu, M.; Mila, I.; Khoudi, H.; Bernadac, A.; Bouzayen, M.; Pirrello, J. Ethylene Response Factors (ERF) are differentially regulated by different abiotic stress types in tomato plants. Plant Sci. 2018, 274, 137–145. [Google Scholar] [CrossRef]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta Gene Regul. Mech. 2012, 1819, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sunarti, S.; Kissoudis, C.; Visser, R.G.F.; van der Linden, C.G. The role of tomato WRKY genes in plant responses to combined abiotic and biotic stresses. Front. Plant Sci. 2018, 9, 801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, G.; Sharma, N.; Pankaj Sahu, P.; Prasad, M. Chromatin-Based Epigenetic Regulation of Plant Abiotic Stress Response. Curr. Genom. 2016, 17, 490–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrella, R.; Caselli, F.; Roig-Villanova, I.; Vignati, V.; Chiara, M.; Ezquer, I.; Tadini, L.; Kater, M.M.; Gregis, V. BPC transcription factors and a Polycomb Group protein confine the expression of the ovule identity gene SEEDSTICK in Arabidopsis. Plant J. 2020, tpj.14673. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; James, D.; Reddy, M.K. Omics Technologies for Abiotic Stress Tolerance in Plants: Current Status and Prospects. In Recent Approaches in Omics for Plant Resilience to Climate Change; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–34. [Google Scholar]
- De Lorenzo, G.; Ferrari, S.; Giovannoni, M.; Mattei, B.; Cervone, F. Cell wall traits that influence plant development, immunity, and bioconversion. Plant J. 2019, 97, 134–147. [Google Scholar] [CrossRef]
- Blanc, N.F.; Mortimer, J.; Dupree, P. A Transcriptomic Analysis of Xylan Mutants Does Not Support the Existence of A Secondary Cell Wall Integrity System in Arabidopsis. Front. Plant Sci. 2018, 9, 384. [Google Scholar] [CrossRef] [Green Version]
- Reem, N.T.; Chen, H.-Y.; Hur, M.; Zhao, X.; Wurtele, E.S.; Li, X.; Li, L.; Zabotina, O. Comprehensive transcriptome analyses correlated with untargeted metabolome reveal differentially expressed pathways in response to cell wall alterations. Plant Mol. Biol. 2018, 96, 509–529. [Google Scholar] [CrossRef]
- Voxeur, A.; Höfte, H. Cell wall integrity signaling in plants: “To grow or not to grow that’s the question”. Glycobiology 2016, 26, 950–960. [Google Scholar] [CrossRef]
- Wolf, S. Plant cell wall signalling and receptor-like kinases. Biochem. J. 2017, 474, 471–492. [Google Scholar] [CrossRef]
- Joshi, R.; Singla-Pareek, S.L.; Pareek, A. Engineering abiotic stress response in plants for biomass production. J. Biol. Chem. 2018, 293, 5035–5043. [Google Scholar] [CrossRef] [Green Version]
- Swetha, C.; Basu, D.; Pachamuthu, K.; Tirumalai, V.; Nair, A.; Prasad, M.; Shivaprasad, P.V. Major domestication-related phenotypes in indica rice are due to loss of miRNA-mediated laccase silencing. Plant Cell 2018, 30, 2649–2662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boavida, L.C. Plant Cell Wall Composition: Does Ploidy Matter? Plant Physiology. 2019, 179, 16–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuskan, G.A.; DiFazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A.; et al. The Genome of Black Cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [PubMed] [Green Version]
- Brunner, P.C.; Keller, N.; McDonald, B.A. Wheat Domestication Accelerated Evolution and Triggered Positive Selection in the β-Xylosidase Enzyme of Mycosphaerella graminicola. PLoS ONE 2009, 4, e7884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voiniciuc, C.; Pauly, M.; Usadel, B. Monitoring polysaccharide dynamics in the plant cell wall. Plant Physiol. 2018, 176, 2590–2600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dardelle, F.; Le Mauff, F.; Lehner, A.; Loutelier-Bourhis, C.; Bardor, M.; Rihouey, C.; Causse, M.; Lerouge, P.; Driouich, A.; Mollet, J.C. Pollen tube cell walls of wild and domesticated tomatoes contain arabinosylated and fucosylated xyloglucan. Ann. Bot. 2015, 115, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Dehors, J.; Mareck, A.; Kiefer-Meyer, M.C.; Menu-Bouaouiche, L.; Lehner, A.; Mollet, J.C. Evolution of cell wall polymers in tip-growing land plant gametophytes: Composition, distribution, functional aspects and their remodeling. Front. Plant Sci. 2019, 10, 441. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. Does Enhanced Photosynthesis Enhance Growth? Lessons Learned from CO2 Enrichment Studies. Plant Physiol. 2011, 155, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, X.; Chen, D.; Cui, H.; Ge, Q. Overestimated climate warming and climate variability due to spatially homogeneous CO2 in climate modeling over the Northern Hemisphere since the mid-19th century. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Rae, A.M.; Tricker, P.J.; Bunn, S.M.; Taylor, G. Adaptation of tree growth to elevated CO2: Quantitative trait loci for biomass in Populus. New Phytol. 2007, 175, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Boex-Fontvieille, E.; Davanture, M.; Jossier, M.; Zivy, M.; Hodges, M.; Tcherkez, G. Photosynthetic activity influences cellulose biosynthesis and phosphorylation of proteins involved therein in Arabidopsis leaves. J. Exp. Bot. 2014, 65, 4997–5010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-H.; Shim, B.S.; Kim, H.S.; Lee, Y.-J.; Min, S.-K.; Jang, D.; Abas, Z.; Kim, J. Review of nanocellulose for sustainable future materials. Int. J. Precis. Eng. Manuf. Technol. 2015, 2, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caspar, T.; Huber, S.C.; Somerville, C. Alterations in Growth, Photosynthesis, and Respiration in a Starchless Mutant of Arabidopsis thaliana (L.) Deficient in Chloroplast Phosphoglucomutase Activity. Plant Physiol. 1985, 79, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Hanson, K.R.; McHale, N.A. A Starchless Mutant of Nicotiana sylvestris Containing a Modified Plastid Phosphoglucomutase. Plant Physiol. 1988, 88, 838–844. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-P.; Caspar, T.; Somerville, C.R.; Preiss, J. A Starch Deficient Mutant of Arabidopsis thaliana with Low ADPglucose Pyrophosphorylase Activity Lacks One of the Two Subunits of the Enzyme. Plant Physiol. 1988, 88, 1175–1181. [Google Scholar] [CrossRef] [Green Version]
- Ragel, P.; Streb, S.; Feil, R.; Sahrawy, M.; Annunziata, M.G.; Lunn, J.E.; Zeeman, S.; Mérida, Á. Loss of Starch Granule Initiation Has a Deleterious Effect on the Growth of Arabidopsis Plants Due to an Accumulation of ADP-Glucose. Plant Physiol. 2013, 163, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Bahaji, A.; Almagro, G.; Ezquer, I.; Gámez-Arcas, S.; Sánchez-López, Á.M.; Muñoz, F.J.; Barrio, R.J.; Sampedro, M.C.; De Diego, N.; Spíchal, L.; et al. Plastidial phosphoglucose isomerase is an important determinant of seed yield through involvement in gibberellin-mediated reproductive development and biosynthesis of storage reserves in Arabidopsis. Plant Cell 2018, 30, 2082–2098. [Google Scholar] [CrossRef] [Green Version]
- Miura, K.; Hasegawa, P.M. Sumoylation and other ubiquitin-like post-translational modifications in plants. Trends Cell Biol. 2010, 20, 223–232. [Google Scholar] [CrossRef]
- Liu, Y.-B.; Lu, S.-M.; Zhang, J.-F.; Liu, S.; Lu, Y.-T. A xyloglucan endotransglucosylase/hydrolase involves in growth of primary root and alters the deposition of cellulose in Arabidopsis. Planta 2007, 226, 1547–1560. [Google Scholar] [CrossRef]
- Sasidharan, R.; Chinnappa, C.C.; Staal, M.; Elzenga, J.T.M.; Yokoyama, R.; Nishitani, K.; Voesenek, L.A.C.J.; Pierik, R. Light Quality-Mediated Petiole Elongation in Arabidopsis during Shade Avoidance Involves Cell Wall Modification by Xyloglucan Endotransglucosylase/Hydrolases. Plant Physiol. 2010, 154, 978–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; McFarlane, H.E.; Persson, S. The impact of abiotic factors on cellulose synthesis. J. Exp. Bot. 2016, 67, 543–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Hong, X.; Zhang, H.; Wang, Y.; Li, X.; Zhu, J.-K.; Gong, Z. Disruption of the cellulose synthase gene, AtCesA8/IRX1, enhances drought and osmotic stress tolerance in Arabidopsis. Plant J. 2005, 43, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K.J.; Lee, B.-H.; Dellinger, M.; Cui, X.; Zhang, C.; Wu, S.; Nothnagel, E.A.; Zhu, J.-K.J. A cellulose synthase-like protein is required for osmotic stress tolerance in Arabidopsis. Plant J. 2010, 63, 128–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; He, H.; Fang, L.; Zhang, A. Pectin methylesterase31 positively regulates salt stress tolerance in Arabidopsis. Biochem. Biophys. Res. Commun. 2018, 496, 497–501. [Google Scholar] [CrossRef]
- Jithesh, M.N.; Wally, O.S.D.; Manfield, I.; Critchley, A.T.; Hiltz, D.; Prithiviraj, B. Analysis of Seaweed Extract-induced Transcriptome Leads to Identification of a Negative Regulator of Salt Tolerance in Arabidopsis. HortScience 2012, 47, 704–709. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.C.; Bulgakov, V.P.; Jinn, T.L. Pectin methylesterases: Cell wall remodeling proteins are required for plant response to heat stress. Front. Plant Sci. 2018, 871, 1612. [Google Scholar] [CrossRef] [Green Version]
- Wormit, A.; Usadel, B. The Multifaceted Role of Pectin Methylesterase Inhibitors (PMEIs). Int. J. Mol. Sci. 2018, 19, 2878. [Google Scholar] [CrossRef] [Green Version]
- Philippe, F.; Pelloux, J.; Rayon, C. Plant pectin acetylesterase structure and function: New insights from bioinformatic analysis. BMC Genom. 2017, 18, 456. [Google Scholar] [CrossRef] [Green Version]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef] [Green Version]
- An, P.; Li, X.; Zheng, Y.; Matsuura, A.; Abe, J.; Eneji, A.E.; Tanimoto, E.; Inanaga, S. Effects of NaCl on Root Growth and Cell Wall Composition of Two Soya bean Cultivars with Contrasting Salt Tolerance. J. Agron. Crop Sci. 2014, 200, 212–218. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Z.; Song, X.; Xu, J.; Jiang, C.; Zhao, Y.; Ma, C.; Zhang, H. Transcriptomic profiling revealed an important role of cell wall remodeling and ethylene signaling pathway during salt acclimation in Arabidopsis. Plant Mol. Biol. 2014, 86, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Abuqamar, S.; Ajeb, S.; Sham, A.; Enan, M.R.; Iratni, R. A mutation in the expansin-like A2gene enhances resistance to necrotrophic fungi and hypersensitivity to abiotic stress in Arabidopsis thaliana. Mol. Plant Pathol. 2013, 14, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Han, Y.; Han, S.; Ban, Q.; He, Y.; Jin, M.; Rao, J. Overexpression of persimmon DkXTH1 enhanced tolerance to abiotic stress and delayed fruit softening in transgenic plants. Plant Cell Rep. 2017, 36, 583–596. [Google Scholar] [CrossRef]
- Choi, J.Y.; Seo, Y.S.; Kim, S.J.; Kim, W.T.; Shin, J.S. Constitutive expression of CaXTH3, a hot pepper xyloglucan endotransglucosylase/hydrolase, enhanced tolerance to salt and drought stresses without phenotypic defects in tomato plants (Solanum lycopersicum cv. Dotaerang). Plant Cell Rep. 2011, 30, 867–877. [Google Scholar] [CrossRef]
- Cho, S.K.; Kim, J.E.; Park, J.-A.; Eom, T.J.; Kim, W.T. Constitutive expression of abiotic stress-inducible hot pepperCaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenicArabidopsisplants. FEBS Lett. 2006, 580, 3136–3144. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Sa, G.; Sun, J.; Shen, Z.; Zhao, R.; Ding, M.; Deng, S.; Lu, Y.; Zhang, Y.; Shen, X.; et al. Overexpression of Populus euphratica xyloglucan endotransglucosylase/hydrolase gene confers enhanced cadmium tolerance by the restriction of root cadmium uptake in transgenic tobacco. Environ. Exp. Bot. 2014, 100, 74–83. [Google Scholar] [CrossRef]
- Mareri, L.; Faleri, C.; Romi, M.; Mariani, C.; Cresti, M.; Cai, G. Heat stress affects the distribution of JIM8-labelled arabinogalactan proteins in pistils of Solanum lycopersicum cv Micro-Tom. Acta Physiol. Plant. 2016, 38, 184. [Google Scholar] [CrossRef]
- Mareri, L.; Romi, M.; Cai, G. Arabinogalactan proteins: Actors or spectators during abiotic and biotic stress in plants? Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2018, 153, 173–185. [Google Scholar] [CrossRef]
- Olmos, E.; García De La Garma, J.; Gomez-Jimenez, M.C.; Fernandez-Garcia, N. Arabinogalactan Proteins Are Involved in Salt-Adaptation and Vesicle Trafficking in Tobacco by-2 Cell Cultures. Front. Plant Sci. 2017, 8, 1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Zhao, J. Genome-wide identification, classification, and expression analysis of the arabinogalactan protein gene family in rice (Oryza sativa L.). J. Exp. Bot. 2010, 61, 2647–2668. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, N.; Hernandez, M.; Casado-Vela, J.; Bru, R.; Elortza, F.; Hedden, P.; Olmos, E. Changes to the proteome and targeted metabolites of xylem sap in Brassica oleracea in response to salt stress. Plant Cell Environ. 2011, 34, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K.; Bressan, R.; Hasegawa, P. Loss of arabinogalactan-proteins from the plasma membrane of NaCl-adapted tobacco cells. Planta 1993, 190, 221–226. [Google Scholar] [CrossRef]
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.L.; Kibble, N.A.J.; Bacic, A.; Schultz, C.J. A Fasciclin-Like Arabinogalactan-Protein (FLA) Mutant of Arabidopsis thaliana, fla1, Shows Defects in Shoot Regeneration. PLoS ONE 2011, 6, e25154. [Google Scholar] [CrossRef] [Green Version]
- Zang, L.; Zheng, T.; Chu, Y.; Ding, C.; Zhang, W.; Huang, Q.; Su, X. Genome-Wide Analysis of the Fasciclin-Like Arabinogalactan Protein Gene Family Reveals Differential Expression Patterns, Localization, and Salt Stress Response in Populus. Front. Plant Sci. 2015, 6, 1140. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.-C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The FERONIA Receptor Kinase Maintains Cell-Wall Integrity during Salt Stress through Ca2+ Signaling. Curr. Biol. 2018, 28, 666–675. [Google Scholar] [CrossRef] [Green Version]
- Gonneau, M.; Desprez, T.; Martin, M.; Doblas, V.G.; Bacete, L.; Miart, F.; Sormani, R.; Hématy, K.; Renou, J.; Landrein, B.; et al. Receptor Kinase THESEUS1 Is a Rapid Alkalinization Factor 34 Receptor in Arabidopsis. Curr. Biol. 2018, 28, 2452–2458. [Google Scholar] [CrossRef] [Green Version]
- Van der Does, D.; Boutrot, F.; Engelsdorf, T.; Rhodes, J.; McKenna, J.F.; Vernhettes, S.; Koevoets, I.; Tintor, N.; Veerabagu, M.; Miedes, E.; et al. The Arabidopsis leucine-rich repeat receptor kinase MIK2/LRR-KISS connects cell wall integrity sensing, root growth and response to abiotic and biotic stresses. PLoS Genet. 2017, 13, e1006832. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.R.; Kaldenhoff, R.; Genty, B.; Terashima, I. Resistances along the CO2 diffusion pathway inside leaves. J. Exp. Bot. 2009, 60, 2235–2248. [Google Scholar] [CrossRef] [Green Version]
- Delmer, D.P.; Haigler, C.H. The Regulation of Metabolic Flux to Cellulose, a Major Sink for Carbon in Plants. Metab. Eng. 2002, 4, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Gamage, D.; Thompson, M.; Sutherland, M.; Hirotsu, N.; Makino, A.; Seneweera, S. New insights into the cellular mechanisms of plant growth at elevated atmospheric carbon dioxide concentrations. Plant. Cell Environ. 2018, 41, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Lemon, E.R. (Ed.) CO2 and Plants; CRC Press: Boca Raton, FL, USA, 1983. [Google Scholar]
- Koike, T.; Kitao, M.; Hikosaka, K.; Agathokleous, E.; Watanabe, Y.; Watanabe, M.; Eguchi, N.; Funada, R. Photosynthetic and Photosynthesis-Related Responses of Japanese Native Trees to CO2: Results from Phytotrons, Open-Top Chambers, Natural CO2 Springs, and Free-Air CO2 Enrichment. In The Leaf: A Platform for Performing Photosynthesis; Springer International Publishing: Cham, Switzerland, 2018; pp. 425–449. [Google Scholar]
- Zhu, C.; Ziska, L.; Zhu, J.; Zeng, Q.; Xie, Z.; Tang, H.; Jia, X.; Hasegawa, T. The temporal and species dynamics of photosynthetic acclimation in flag leaves of rice (Oryza sativa) and wheat (Triticum aestivum) under elevated carbon dioxide. Physiol. Plant. 2012, 145, 395–405. [Google Scholar] [CrossRef]
- Teng, N.; Wang, J.; Chen, T.; Wu, X.; Wang, Y.; Lin, J. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 2006, 172, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, E.; Riikonen, J.; Kaakinen, S.; Holopainen, T.; Vapaavuori, E. Structural characteristics and chemical composition of birch (Betula pendula) leaves are modified by increasing CO2 and ozone. Glob. Chang. Biol. 2005, 11, 732–748. [Google Scholar] [CrossRef]
- Aidar, M.P.M.; Martinez, C.A.; Costa, A.C.; Costa, P.M.F.; Dietrich, S.M.C.; Buckeridge, M.S. Effect of atmospheric CO2 enrichment on the establishment of seedlings of Jatobá, Hymenaea courbaril L. (Leguminosae, Caesalpinioideae). Biota Neotrop. 2002, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Sinha, P.G.; Bhatnagar, A.K. Effect of elevated [CO2] on cell structure and function in seed plants. Clim. Chang. Environ. Sustain. 2014, 2, 69. [Google Scholar] [CrossRef]
- Kim, K.; Labbé, N.; Warren, J.M.; Elder, T.; Rials, T.G. Chemical and anatomical changes in Liquidambar styraciflua L. xylem after long term exposure to elevated CO2. Environ. Pollut. 2015, 198, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Gibeaut, D.M.; Cramer, G.R.; Seemann, J.R. Growth, cell walls, and UDP-Glc dehydrogenase activity of Arabidopsis thaliana grown in elevated carbon dioxide. J. Plant Physiol. 2001, 158, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Pérez-López, U.; Robredo, A.; Lacuesta, M.; Muñoz-Rueda, A.; Mena-Petite, A. Atmospheric CO2 concentration influences the contributions of osmolyte accumulation and cell wall elasticity to salt tolerance in barley cultivars. J. Plant Physiol. 2010, 167, 15–22. [Google Scholar] [CrossRef]
- Ookawara, R.; Satoh, S.; Yoshioka, T.; Ishizawa, K. Expression of α-expansin and xyloglucan endotransglucosylase/ hydrolase genes associated with shoot elongation enhanced by anoxia, ethylene and carbon dioxide in arrowhead (Sagittaria pygmaea Miq.) tubers. Ann. Bot. 2005, 96, 693–702. [Google Scholar] [CrossRef] [Green Version]
- May, P.; Liao, W.; Wu, Y.; Shuai, B.; Richard McCombie, W.; Zhang, M.Q.; Liu, Q.A. The effects of carbon dioxide and temperature on microRNA expression in Arabidopsis development. Nat. Commun. 2013, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Sharma, A.; Mishra, M.; Mishra, R.K.; Chowdhuri, D.K. Heat shock proteins in toxicology: How close and how far? Life Sci. 2010, 86, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Gou, J.; Yordanov, Y.; Zhang, H.; Thakur, R.; Jones, W.; Burton, A. Global transcriptomic profiling of aspen trees under elevated [CO2] to identify potential molecular mechanisms responsible for enhanced radial growth. J. Plant Res. 2013, 126, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Nobel, P.S. Doubling the CO2 Concentration Enhanced the Activity of Carbohydrate-Metabolism Enzymes, Source Carbohydrate Production, Photoassimilate Transport, and Sink Strength for Opuntia ficus-indica. Plant Physiol. 1996, 110, 893–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frary, A. Plant Physiology and Development Plant Physiology and Development edited by Lincoln Taiz, Eduardo Zeiger, Ian Max Moller, and Angus Murphy. Rhodora 2015, 117, 397–399. [Google Scholar] [CrossRef]
- Wu, Y.; Cosgrove, D.J. Adaptation of Roots to Low Water Potentials by Changes in Cell Wall Extensibility and Cell Wall Proteins. J. Exp. Bot. 2000, 1543–1553. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.P.; Vicré-Gibouin, M.; Farrant, J.M.; Driouich, A. Adaptations of higher plant cell walls to water loss: Drought vs desiccation. Physiol. Plant. 2008, 134, 237–245. [Google Scholar] [CrossRef]
- Rao, X.; Dixon, R.A. Brassinosteroid Mediated Cell Wall Remodeling in Grasses under Abiotic Stress. Front. Plant Sci. 2017, 8, 806. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Yang, C.; Wang, X. Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in Arabidopsis. J. Exp. Bot. 2011, 62, 4495–4506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Rodríguez, C.; Ketelaar, K.; Schneider, R.; Villalobos, J.A.; Somerville, C.R.; Persson, S.; Wallace, I.S. BRASSINOSTEROID INSENSITIVE2 negatively regulates cellulose synthesis in Arabidopsis by phosphorylating cellulose synthase 1. Proc. Natl. Acad. Sci. USA 2017, 114, 3533–3538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Placido, D.F.; Campbell, M.T.; Folsom, J.J.; Cui, X.; Kruger, G.R.; Baenziger, P.S.; Walia, H. Introgression of Novel Traits from a Wild Wheat Relative Improves Drought Adaptation in Wheat. Plant Physiol. 2013, 161, 1806–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marowa, P.; Ding, A.; Kong, Y. Expansins: Roles in plant growth and potential applications in crop improvement. Plant Cell Rep. 2016, 35, 949–965. [Google Scholar] [CrossRef] [Green Version]
- Yennawar, N.H.; Li, L.-C.; Dudzinski, D.M.; Tabuchi, A.; Cosgrove, D.J. Crystal structure and activities of EXPB1 (Zea m 1), a beta-expansin and group-1 pollen allergen from maize. Proc. Natl. Acad. Sci. USA 2006, 103, 14664–14671. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, L.; Hao, W.; Zhang, L.; Liu, Y.; Chen, L. Expression of Two α-Type Expansins from Ammopiptanthus nanus in Arabidopsis thaliana Enhance Tolerance to Cold and Drought Stresses. Int. J. Mol. Sci. 2019, 20, 5255. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Park, Y.B.; Cosgrove, D.J.; Hong, M. Cellulose-Pectin Spatial Contacts Are Inherent to Never-Dried Arabidopsis Primary Cell Walls: Evidence from Solid-State Nuclear Magnetic Resonance. Plant Physiol. 2015, 168, 871–884. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Xu, X.; Shi, Y.; Xu, J.; Huang, B. Transgenic tobacco plants overexpressing a grass PpEXP1 gene exhibit enhanced tolerance to heat stress. PLoS ONE 2014, 9, e100792. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhao, J.; Li, X.; Qin, L.; Yan, X.; Liao, H. A soybean β-expansin gene GmEXPB2 intrinsically involved in root system architecture responses to abiotic stresses. Plant J. 2011, 66, 541–552. [Google Scholar] [CrossRef]
- Dai, F.; Zhang, C.; Jiang, X.; Kang, M.; Yin, X.; Lü, P.; Zhang, X.; Zheng, Y.; Gao, J. RhNAC2 and RhEXPA4 Are Involved in the Regulation of Dehydration Tolerance during the Expansion of Rose Petals. Plant Physiol. 2012, 160, 2064–2082. [Google Scholar] [CrossRef] [Green Version]
- An, S.H.; Sohn, K.H.; Choi, H.W.; Hwang, I.S.; Lee, S.C.; Hwang, B.K. Pepper pectin methylesterase inhibitor protein CaPMEI1 is required for antifungal activity, basal disease resistance and abiotic stress tolerance. Planta 2008, 228, 61–78. [Google Scholar] [CrossRef] [Green Version]
- Leucci, M.R.; Lenucci, M.S.; Piro, G.; Dalessandro, G. Water stress and cell wall polysaccharides in the apical root zone of wheat cultivars varying in drought tolerance. J. Plant Physiol. 2008, 165, 1168–1180. [Google Scholar] [CrossRef]
- Yang, W.; Ruan, M.; Xiang, M.; Deng, A.; Du, J.; Xiao, C. Overexpression of a pectin methylesterase gene PtoPME35 from Populus tomentosa influences stomatal function and drought tolerance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2019. [Google Scholar] [CrossRef]
- Haigler, C.H.; Betancur, L.; Stiff, M.R.; Tuttle, J.R. Cotton fiber: A powerful single-cell model for cell wall and cellulose research. Front. Plant Sci. 2012, 3, 104. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Yañez, Á.; Beltrán, D.; Campano-Romero, C.; Molinett, S.; Herrera, R.; Moya-León, M.A.; Morales-Quintana, L. Glycosylation is important for FcXTH1 activity as judged by its structural and biochemical characterization. Plant Physiol. Biochem. 2017, 119, 200–210. [Google Scholar] [CrossRef]
- Campbell, P.; Braam, J. In vitro activities of four xyloglucan endotransglycosylases from Arabidopsis. Plant J. 1999, 18, 371–382. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Catalysts of plant cell wall loosening. F1000Research 2016, 5, 119. [Google Scholar] [CrossRef] [Green Version]
- Vissenberg, K.; Oyama, M.; Osato, Y.; Yokoyama, R.; Verbelen, J.-P.; Nishitani, K. Differential Expression of AtXTH17, AtXTH18, AtXTH19 and AtXTH20 Genes in Arabidopsis Roots. Physiological Roles in Specification in Cell Wall Construction. Plant Cell Physiol. 2005, 46, 192–200. [Google Scholar] [CrossRef]
- Franková, L.; Fry, S.C. Biochemistry and physiological roles of enzymes that ‘cut and paste’ plant cell-wall polysaccharides. J. Exp. Bot. 2013, 64, 3519–3550. [Google Scholar] [CrossRef] [Green Version]
- Landi, S.; Hausman, J.-F.; Guerriero, G.; Esposito, S. Poaceae vs. Abiotic Stress: Focus on Drought and Salt Stress, Recent Insights and Perspectives. Front. Plant Sci. 2017, 8, 1214. [Google Scholar] [CrossRef] [Green Version]
- Seki, M.; Ishida, J.; Narusaka, M.; Fujita, M.; Nanjo, T.; Umezawa, T.; Kamiya, A.; Nakajima, M.; Enju, A.; Sakurai, T.; et al. Monitoring the expression pattern of around 7000 Arabidopsis genes under ABA treatments using a full-length cDNA microarray. Funct. Integr. Genom. 2002, 2, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Tseng, I.-C.; Hong, C.-Y.; Yu, S.-M.; Ho, T.-H.D. Abscisic Acid- and Stress-Induced Highly Proline-Rich Glycoproteins Regulate Root Growth in Rice. Plant Physiol. 2013, 163, 118–134. [Google Scholar] [CrossRef] [Green Version]
- Rabello, A.R.; Guimaraes, C.M.; Rangel, P.H.N.; da Silva, F.R.; Seixas, D.; de Souza, E.; Brasileiro, A.C.M.; Spehar, C.R.; Ferreira, M.E.; Mehta, A. Identification of drought-responsive genes in roots of upland rice (Oryza sativa L). BMC Genom. 2008, 9, 485. [Google Scholar] [CrossRef] [Green Version]
- Bäurle, I. Plant Heat Adaptation: Priming in response to heat stress. F1000Research 2016, 5, 694. [Google Scholar] [CrossRef]
- Park, C.-J.; Seo, Y.-S. Heat Shock Proteins: A Review of the Molecular Chaperones for Plant Immunity. Plant Pathol. J. 2015, 31, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Xiang, Y. An Overview of Biomembrane Functions in Plant Responses to High-Temperature Stress. Front. Plant Sci. 2018, 9, 915. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, R.I. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008, 22, 1427–1438. [Google Scholar] [CrossRef] [Green Version]
- Lima, R.B.R.; dos Santos, T.B.; Vieira, L.G.E.; de Lourdes Lúcio Ferrarese, M.; Ferrarese-Filho, O.; Donatti, L.; Boeger, M.R.T.; de Oliveira Petkowicz, C.L. Heat stress causes alterations in the cell-wall polymers and anatomy of coffee leaves (Coffea arabica L.). Carbohydr. Polym. 2013, 93, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.A.; Lim, C.J.; Hong, J.K.; Park, C.Y.; Cheong, Y.H.; Chung, W.S.; Lee, K.O.; Lee, S.Y.; Cho, M.J.; Lim, C.O. Identification of cell wall genes modified by a permissive high temperature in Chinese cabbage. Plant Sci. 2006, 171, 175–182. [Google Scholar] [CrossRef]
- Edreva, A.; Sotirova, V.; Georgieva, I.D.; Stoimenova, E.; Rodeva, R.; Bogatzevska, N. Differential expression of β-glucosidase in tomato—Stress stimuli systems. Acta Physiol. Plant. 2000, 22, 274–277. [Google Scholar] [CrossRef]
- Konno, H.; Yamasaki, Y.; Sugimoto, M.; Takeda, K. Differential changes in cell wall matrix polysaccharides and glycoside-hydrolyzing enzymes in developing wheat seedlings differing in drought tolerance. J. Plant Physiol. 2008, 165, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M.; Boorsma, A.; De Groot, P.W.J. Cell wall construction inSaccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Fan, C.; Yang, Q.; Li, X.; Wan, B.; Dong, Y.; Wang, X.; Zhou, Y. Identification of heat responsive genes in Brassica napus Siliques at the seed-filling stage through transcriptional profiling. PLoS ONE 2014, 9, e101914. [Google Scholar] [CrossRef]
- Carvalho, C.P.; Hayashi, A.H.; Braga, M.R.; Nievola, C.C. Biochemical and anatomical responses related to the in vitro survival of the tropical bromeliad Nidularium minutum to low temperatures. Plant Physiol. Biochem. 2013, 71, 144–154. [Google Scholar] [CrossRef]
- Huang, Y.C.; Wu, H.C.; Wang, Y.D.; Liu, C.H.; Lin, C.C.; Luo, D.L.; Jinn, T.L. PECTIN METHYLESTERASE34 contributes to heat tolerance through its role in promoting stomatal movement. Plant Physiol. 2017, 174, 748–763. [Google Scholar] [CrossRef]
- Wu, H.-C.; Hsu, S.-F.; Luo, D.-L.; Chen, S.-J.; Huang, W.-D.; Lur, H.-S.; Jinn, T.-L. Recovery of heat shock-triggered released apoplastic Ca2+ accompanied by pectin methylesterase activity is required for thermotolerance in soybean seedlings. J. Exp. Bot. 2010, 61, 2843–2852. [Google Scholar] [CrossRef] [Green Version]
- Shan, H.; Zhang, N.; Liu, C.; Xu, G.; Zhang, J.; Chen, Z.; Kong, H. Patterns of gene duplication and functional diversification during the evolution of the AP1/SQUA subfamily of plant MADS-box genes. Mol. Phylogenet. Evol. 2007, 44, 26–41. [Google Scholar] [CrossRef]
- Xu, J.; Tian, J.; Belanger, F.C.; Huang, B. Identification and characterization of an expansin gene AsEXP1 associated with heat tolerance in C3 Agrostis grass species. J. Exp. Bot. 2007, 58, 3789–3796. [Google Scholar] [CrossRef] [Green Version]
- Schopfer, P.; Liszkay, A. Plasma membrane-generated reactive oxygen intermediates and their role in cell growth of plants. BioFactors 2006, 28, 73–81. [Google Scholar] [CrossRef]
- Xu, W.; Campbell, P.; Vargheese, A.K.; Braam, J. The Arabidopsis XET-related gene family: Environmental and hormonal regulation of expression. Plant J. 1996, 9, 879–889. [Google Scholar] [CrossRef]
- Iurlaro, A.; De Caroli, M.; Sabella, E.; De Pascali, M.; Rampino, P.; De Bellis, L.; Perrotta, C.; Dalessandro, G.; Piro, G.; Fry, S.C.; et al. Drought and Heat Differentially Affect XTH Expression and XET Activity and Action in 3-Day-Old Seedlings of Durum Wheat Cultivars with Different Stress Susceptibility. Front Plant Sci. 2016, 7, 1686. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.J.; Roberts, K. The biology of arabinogalactan proteins. Annu. Rev. Plant Biol. 2007, 58, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Showalter, A.M. Immunolocalization of extensin and potato tuber lectin in carrot, tomato and potato. Physiol. Plant. 1996, 97, 708–718. [Google Scholar] [CrossRef]
- Baroja-Fernandez, E.; Etxeberria, E.; Muñoz, F.J.; Morán-Zorzano, M.T.; Alonso-Casajús, N.; Gonzalez, P.; Pozueta-Romero, J. An important pool of sucrose linked to starch biosynthesis is taken up by endocytosis in heterotrophic cells. Plant Cell Physiol. 2006, 47, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Bihmidine, S.; Julius, B.T.; Dweikat, I.; Braun, D.M. Tonoplast Sugar Transporters (SbTSTs) putatively control sucrose accumulation in sweet sorghum stems. Plant Signal. Behav. 2016, 11, e1117721. [Google Scholar] [CrossRef] [Green Version]
- Durand, M.; Mainson, D.; Porcheron, B.; Maurousset, L.; Lemoine, R.; Pourtau, N. Carbon source–sink relationship in Arabidopsis thaliana: The role of sucrose transporters. Planta 2018, 247, 587–611. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, M.; Kiegle, E.; Leo, G.; Ezquer, I. Carbohydrate reserves and seed development: An overview. Plant Reprod. 2018, 31, 263–290. [Google Scholar] [CrossRef]



| Stress | Polymers | Species | Gene | Reference |
|---|---|---|---|---|
| Salinity stress | Cellulose | Arabidopsis thaliana | CesA | Chen et al., 2005; Zhu et al., 2010 [108,109] |
| Pectins | Arabidopsis thaliana | PME | Yan et al., 2018; Jithesh et al., 2012 [110,111] | |
| Arabidopsis thaliana | PAE | Philippe et al., 2017 [114] | ||
| Glycine max | PAE | Tenhaken, 2015; An et al., 2014 [115,116] | ||
| Hemicellulose | Arabidopsis thaliana | XTH | Le Gall et al., 2015; Tenhaken, 2015; Cho et al., 2006; Choi et al., 2011; Han et al., 2017 [115,119,120,121,122] | |
| Solanum lycopersicum | XTH | Han et al., 2017 [120] | ||
| Capsicum annuum | XTH | Choi et al., 2011 [121] | ||
| Nicotiana tabacum | XTH | Han et al., 2014 [123] | ||
| Cell wall proteins | Arabidopsis thaliana | EXP | Shen et al., 2014; Abuqamar et al., 2013 [117,118] | |
| Arabidopsis thaliana | EXLA | Tenhaken, 2015; Abuqamar et al., 2013 [115,118] | ||
| Arabidopsis thaliana | GPRs | Shen et al., 2014 [117] | ||
| Arabidopsis thaliana | AGPs | Shen et al., 2014 [117] | ||
| Nicotiana tabacum | AGPs | Olmos et al., 2017; Zhu et al., 1993; Zhu et al., 2002; Lamport et al., 2006 [68,126,129,130] | ||
| Oryza sativa | AGPs | Ma & Zhao, 2010 [127] | ||
| Brassica oleracea | AGPs | Fernandez-Garcia et al., 2011 [128] | ||
| Arabidopsis thaliana | FLA | Johnson et al., 2011 [131] | ||
| Populus trichocarpa | FLA | Zang et al., 2015 [132] | ||
| Elevated atmospheric CO2 | Cellulose | Hymenaea courbaril | CesA | Aidar et al., 2002 [144] |
| Arabidopsis thaliana | CesA | Teng et al., 2006 [142] | ||
| Quercus petraea | CesA | Koike et al., 2018 [140] | ||
| Hymenaea courbaril | CesA | Aidar et al., 2002 [144] | ||
| Arabidopsis thaliana | CesA | Teng et al., 2006 [142] | ||
| Pectins | Populus tremuloides | PME | Le Gall et al., 2015 [119] | |
| Hemicellulose | Plantago media | XET | Sharma., 2014 [145] | |
| Liquidambar styraciflua | XTH | Kim et al., 2015 [146] | ||
| Populus tremuloides | XTH | Gupta et al., 2010 [151] | ||
| Betula pendula | XTH | Oksanen et al., 2005 [143] | ||
| Hordeum vulgare | XTH | Le Gall et al., 2015; Ookawara et al., 2005 [119,149] | ||
| Cell wall proteins | Hordeum vulgare | EXP | Le Gall et al., 2015; Ookawara et al., 2005 [119,149] | |
| Liquidambar styraciflua | EXP | Kim et al., 2015 [146] | ||
| Populus tremuloides | EXP | Gupta et al., 2010 [151] | ||
| Drought stress | Cellulose | Arabidopsis thaliana | CesA | Chen et al., 2005, Xie et al., 2011 [108,158] |
| Pectins | Arabidopsis thaliana | PME | Wormit & Usadel, 2018; Yang et al., 2019 [113,170] | |
| Hemicellulose | Arabidopsis thaliana | XTH | Cho et al., 2006 [122] | |
| Solanum lycopersicum | XTH | Choi et al., 2011 [121] | ||
| Arabidopsis thaliana | EXP | Liu et al., 2019; Dai et al., 2012; Han et al., 2017 [120,163,167] | ||
| Nicotiana tabacum | EXP | Xu et al., 2014 [165] | ||
| Glycine max | EXP | Guo et al., 2011 [166] | ||
| Cell wall proteins | Oryza sativa | AGP | Tseng et al., 2013 [179] | |
| Arabidopsis thaliana | AGP | Seki et al., 2002 [178] | ||
| Heat stress | Cellulose | Brassica rapa | CesA | Yang et al., 2006 [186] |
| Pectins | Brassica napus | PME | Yu et al., 2014 [190] | |
| Coffea arabica | PME | Lima et al., 2013 [185] | ||
| Nidularium minutum | PME | Carvalho et al., 2013 [191] | ||
| Glycine max | PME | Wu et al., 2010 [193] | ||
| Arabidopsis thaliana | PME | Huang et al., 2017 [192] | ||
| Hemicellulose | Brassica rapa | XTH | Yang et al., 2006 [186] | |
| Triticum aestivum | XET | Iurlaro et al., 2016 [198] | ||
| Coffea arabica | XTH | Lima et al., 2013 [185] | ||
| Triticum aestivum | XTH | Iurlaro et al., 2016 [198] | ||
| Arabidopsis thaliana | XTH | Xu et al., 1996 [197] | ||
| Cell wall proteins | Brassica rapa | EXP | Yang et al., 2006 [186] | |
| Brassica rapa | EXT | Yang et al., 2006 [186] | ||
| Agrostis scabra | EXP | Xu et al., 2007 [195] | ||
| Nicotiana tabacum | EXP | Xu et al., 2014 [165] | ||
| Solanum lycopersicum | AGP | Li and Showalter 1996; Mareri et al., 2016 [54,124] | ||
| Coffea arabica | AGP | Lima et al., 2013 [185] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezquer, I.; Salameh, I.; Colombo, L.; Kalaitzis, P. Plant Cell Walls Tackling Climate Change: Biotechnological Strategies to Improve Crop Adaptations and Photosynthesis in Response to Global Warming. Plants 2020, 9, 212. https://doi.org/10.3390/plants9020212
Ezquer I, Salameh I, Colombo L, Kalaitzis P. Plant Cell Walls Tackling Climate Change: Biotechnological Strategies to Improve Crop Adaptations and Photosynthesis in Response to Global Warming. Plants. 2020; 9(2):212. https://doi.org/10.3390/plants9020212
Chicago/Turabian StyleEzquer, Ignacio, Ilige Salameh, Lucia Colombo, and Panagiotis Kalaitzis. 2020. "Plant Cell Walls Tackling Climate Change: Biotechnological Strategies to Improve Crop Adaptations and Photosynthesis in Response to Global Warming" Plants 9, no. 2: 212. https://doi.org/10.3390/plants9020212