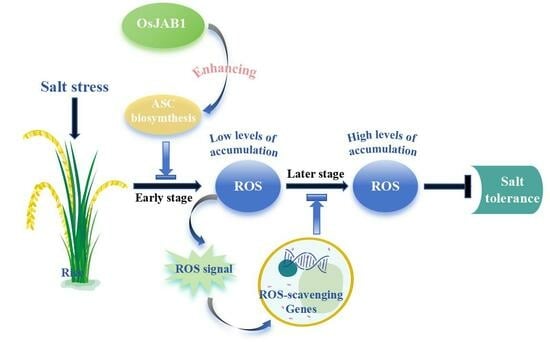
OsJAB1 Positively Regulates Ascorbate Biosynthesis and Negatively Regulates Salt Tolerance Due to Inhibiting Early-Stage Salt-Induced ROS Accumulation in Rice
Abstract
:1. Introduction
2. Results
2.1. The Expression of OsJAB1 (JUN-Activation-Domain-Binding Protein 1) Is Induced by Salt Stress
2.2. OsJAB1 Negatively Regulates Rice Salt Stress Tolerance
2.3. OsJAB1 Positively Regulates Rice Asc Biosynthesis
2.4. OsJAB1 Affects the Accumulation of Salt-Stress-Induced ROS
2.5. OsJAB1 Impaired the Expression of ROS-Scavenging Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Condition
4.2. The Generation of Transgenic Rice
4.3. Analysis of Salt Stress Tolerance
4.4. Quantitative Real-Time PCR (qPCR) Assay
4.5. Asc Content Measurement
4.6. H2O2 Content Measurement
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adnan, M.; Fahad, S.; Muhammad, Z.; Shahen, S.; Ishaq, A.M.; Subhan, D.; Zafar-ul-Hye, M.; Martin, L.B.; Raja, M.M.N.; Beena, S.; et al. Coupling Phosphate-Solubilizing Bacteria with Phosphorus Supplements Improve Maize Phosphorus Acquisition and Growth under Lime Induced Salinity Stress. Plants 2020, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.; Rivero, R.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.; Fichman, Y.; Breusegem, F.V. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Peláez-Vico, M.A.; Fichman, Y.; Zandalinas, S.I.; Breusegem, F.V.; Karpiński, S.M.; Mittler, R. ROS and redox regulation of cell-to-cell and systemic signaling in plants during stress. Free Radic. Biol. Med. 2022, 193, 354–362. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS Regulation During Abiotic Stress Responses in Crop Plants. Front. Plant Sci. 2015, 6, 690–695. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Gallie, D.R. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot. 2013, 64, 433–443. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Z.; Zhu, L.; Wang, J.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Wang, Y.; Zhang, Z. The multiple roles of ascorbate in the abiotic stress response of plants: Antioxidant, cofactor, and regulator. Front. Plant Sci. 2021, 12, 598173. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Khew, C.Y.; Morshed, M.M.; Namasivayam, P.; Napis, S.; Ho, C.L. Overexpression of monodehydroascorbate reductase from a mangrove plant (AeMDHAR) confers salt tolerance on rice. J. Plant Physiol. 2012, 169, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kakan, X.; Yu, Y.; Li, S.; Li, X.; Huang, R.; Wang, J. Ascorbic acid modulation by ABI4 transcriptional repression of VTC2 in the salt tolerance of Arabidopsis. BMC Plant Biol. 2021, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Xu, D.; Li, J.; Deng, X. COP9 signalosome: Discovery, conservation, activity, and function. J. Integr. Plant Biol. 2020, 62, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shah, S.; Xiang, X.; Wang, J.; Deng, Z.; Liu, C.; Zhang, L.; Wu, J.; Edmonds, T.; Jambor, C.; et al. COP9-associated CSN5 regulates exosomal protein deubiquitination and sorting. Am. J. Pathol. 2009, 174, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Gusmaroli, G.; Feng, S.; Deng, X. The Arabidopsis CSN5A and CSN5B subunits are present in distinct COP9 signalosome complexes, and mutations in their JAMM domains exhibit differential dominant negative effects on development. Plant Cell. 2004, 16, 2984–3001. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Zhang, Z.; Quan, R.; Zhang, H.; Ma, L.; Deng, X.; Huang, R. Arabidopsis CSN5B interacts with VTC1 and modulates ascorbic acid synthesis. Plant Cell. 2013, 25, 625–636. [Google Scholar] [CrossRef]
- Li, Y.; Chu, Z.; Luo, J.; Zhou, Y.; Cai, Y.; Lu, Y.; Xia, J.; Kuang, H.; Ye, Z.; Ouyang, B. The C2H2 zinc-finger protein SlZF 3 regulates AsA synthesis and salt tolerance by interacting with CSN5B. Plant Biotechnol. J. 2018, 16, 1201–1213. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kimura, S.; Mori, Y.; Uchiyama, Y.; Ishibashi, T.; Hashimoto, J.; Sakaguchi, K. Interaction between proliferating cell nuclear antigen and JUN-activation-domain-binding protein 1 in the meristem of rice, Oryza sativa L. Planta 2003, 217, 175–183. [Google Scholar] [CrossRef]
- Song, J.; Xing, Y.; Munir, S.; Yu, C.; Song, L.; Li, H.; Wang, T.; Ye, Z. An ATL78-Like RING-H2 finger protein confers abiotic stress tolerance through interacting with RAV2 and CSN5B in tomato. Front. Plant Sci. 2016, 7, 1305. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Guo, Q.; Guo, Q.; Liu, P.; Li, Y.; Wu, C.; Yang, G.; Huang, J.; Zhang, S.; et al. Salt responsive alternative splicing of a RING finger E3 ligase modulates the salt stress tolerance by fine-tuning the balance of COP9 signalosome subunit 5A. PLoS Genet. 2021, 17, e1009898. [Google Scholar] [CrossRef]
- Bonifacio, A.; Martins, M.O.; Ribeiro, C.W.; Fontenele, A.V.; Carvalho, F.E.; Margis-Pinheiro, M.; Silveira, J.A. Role of peroxidases in the compensation of cytosolic ascorbate peroxidase knockdown in rice plants under abiotic stress. Plant Cell Environ. 2011, 34, 1705–1722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Wu, J.; Zheng, X.; Zheng, S.; Sun, X.; Qiu, Q.; Lu, T. Gene knockout study reveals that cytosolic ascorbate peroxidase 2 (OsAPX2) plays a critical role in growth and reproduction in rice under drought, salt and cold stresses. PLoS ONE 2013, 8, e57472. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Bonifacio, A.; Carvalho, F.E.; Andrade, C.M.; Passaia, G.; Schünemann, M.; Maraschin, F.D.S.; Martins, M.O.; Teixeira, F.K.; Rauber, R.; et al. The knockdown of chloroplastic ascorbate peroxidases reveals its regulatory role in the photosynthesis and protection under photo-oxidative stress in rice. Plant Sci. 2014, 214, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.M.; Lin, W.R.; Kao, C.H.; Hong, C.Y. Gene knockout of glutathione reductase 3 results in increased sensitivity to salt stress in rice. Plant Mol. Biol. 2015, 87, 555–564. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, L.; Liu, J.; Cao, Z.; Du, X.; Huang, F.; Pan, G.; Cheng, F. Involvement of CAT in the detoxification of HT-induced ROS burst in rice anther and its relation to pollen fertility. Plant Cell Rep. 2018, 37, 741–757. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.; Kim, J.; Shin, S.; Kwak, S.; Lee, C.; Park, H.; Kim, Y.; Kim, I.; Yoon, S. Over-Expression of Dehydroascorbate Reductase Improves Salt Tolerance, Environmental Adaptability and Productivity in Oryza sativa. Antioxidants 2022, 11, 1077. [Google Scholar] [CrossRef]
- Seong, E.S.; Guo, J.; Kim, Y.H.; Cho, J.H.; Lim, C.K.; Hur, J.H.; Wang, M.H. Regulations of marker genes involved in biotic and abiotic stress by overexpression of the AtNDPK2 gene in rice. Biochem. Biophys. Res. Commun. 2007, 363, 126–132. [Google Scholar] [CrossRef]
- Jiang, W.; Ye, Q.; Wu, Z.; Zhang, Q.; Wang, L.; Liu, J.; Hu, X.; Guo, D.; Wang, X.; Zhang, Z.; et al. Analysis of CAT Gene Family and Functional Identification of OsCAT3 in Rice. Genes 2023, 14, 138. [Google Scholar] [CrossRef]
- Ma, S.; Li, H.; Wang, L.; Li, B.; Wang, Z.; Ma, B.; Ma, F.; Li, M. F-box protein MdAMR1L1 regulates ascorbate biosynthesis in apple by modulating GDP-mannose pyrophosphorylase. Plant Physiol. 2022, 188, 653–669. [Google Scholar] [CrossRef]
- Singh, A.K.; Chamovitz, D.A. Role of Cop9 Signalosome Subunits in the Environmental and Hormonal Balance of Plant. Biomolecules 2019, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and signaling in a higher-CO2 world. Annu. Rev. Plant Biol. 2020, 71, 157–182. [Google Scholar] [CrossRef]
- Shigeoka, S.; Maruta, T. Cellular redox regulation, signaling, and stress response in plants. Biosci. Biotechnol. Biochem. 2014, 78, 1457–1470. [Google Scholar] [CrossRef]
- Chen, G.; Han, H.; Yang, X.; Du, R.; Wang, X. Salt Tolerance of Rice Is Enhanced by the SS3 Gene, Which Regulates Ascorbic Acid Synthesis and ROS Scavenging. Int. J. Mol. Sci. 2023, 23, 10338. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Pantha, S.; Acharya, R.; Ueda, Y.; Wu, L.; Ashrafuzzaman, M.; Ishizaki, T.; Wissuwa, M.; Bulley, S.; Frei, M. Enhanced ascorbate level improves multi-stress tolerance in a widely grown indica rice variety without compromising its agronomic characteristics. J. Plant Physiol. 2019, 240, 152998. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Y.; Liu, W.; Liu, Z. Overexpression of an alfalfa GDP-mannose 3, 5-epimerase gene enhances acid, drought and salt tolerance in transgenic Arabidopsis by increasing ascorbate accumulation. Biotechnol. Lett. 2014, 36, 2331–2341. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Qin, H.; Li, Z.; Liu, H.; Wang, J.; Zhang, H.; Quan, R.; Huang, R.; Zhang, Z. The synthesis of ascorbic acid in rice roots plays an important role in the salt tolerance of rice by scavenging ROS. Int. J. Mol. Sci. 2018, 19, 3347. [Google Scholar] [CrossRef]
- Ma, L.; Qi, W.; Bai, J.; Li, H.; Fang, Y.; Xu, J.; Xu, Y.; Zeng, X.; Pu, Y.; Wang, W.; et al. Genome-Wide Identification and Analysis of the Ascorbate Peroxidase (APX) Gene Family of Winter Rapeseed (Brassica rapa L.) Under Abiotic Stress. Front. Plant Sci. 2022, 12, 2849. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, Y.; Chen, W.; Tang, K.; Zhang, L. Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J. Integr. Plant Biol. 2010, 52, 400–409. [Google Scholar] [CrossRef]
- Qin, H.; Deng, Z.; Zhang, C.; Wang, Y.; Wang, J.; Liu, H.; Zhang, Z.; Huang, R.; Zhang, Z. Rice GDP-mannose pyrophosphorylase OsVTC1-1 and OsVTC1-3 play different roles in ascorbic acid synthesis. Plant Mol. Biol. 2016, 90, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Tanaka, Y.; Ishikawa, T.; Maruta, T. Chloroplast dehydroascorbate reductase and glutathione cooperatively determine the capacity for ascorbate accumulation under photooxidative stress conditions. Plant J. 2023, 114, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Le Martret, B.; Poage, M.; Shiel, K.; Nugent, G.D.; Dix, P.J. Tobacco chloroplast transformants expressing genes encoding dehydroascorbate reductase, glutathione reductase, and glutathione-S-transferase, exhibit altered anti-oxidant metabolism and improved abiotic stress tolerance. Plant Biotechnol. J. 2011, 9, 661–673. [Google Scholar] [CrossRef]
- Yoshida, S.; Tamaoki, M.; Shikano, T.; Nakajima, N.; Ogawa, D.; Ioki, M.; Aono, M.; Kubo, A.; Kamada, H.; Inoue, Y.; et al. Cytosolic dehydroascorbate reductase is important for ozone tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 304–308. [Google Scholar] [CrossRef]
- Pignocchi, C.; Kiddle, G.; Hernández, I.; Foster, S.J.; Asensi, A.; Taybi, T.; Barnes, J.; Foyer, C.H. Ascorbate oxidase-dependent changes in the redox state of the apoplast modulate gene transcript accumulation leading to modified hormone signaling and orchestration of defense processes in tobacco. Plant Physiol. 2006, 141, 423–435. [Google Scholar] [CrossRef]
- Zechmann, B. Compartment-specific importance of ascorbate during environmental stress in plants. Antioxid. Redox Signal. 2018, 29, 1488–1501. [Google Scholar] [CrossRef]
- Chen, C.; Twito, S.; Miller, G. New cross talk between ROS, ABA and auxin controlling seed maturation and germination unraveled in APX6 deficient Arabidopsis seeds. Plant Signal Behav. 2014, 9, e976489. [Google Scholar] [CrossRef]
- Fotopoulos, V.; Sanmartin, M.; Kanellis, A.K. Effect of ascorbate oxidase over-expression on ascorbate recycling gene expression in response to agents imposing oxidative stress. J. Exp. Bot. 2006, 57, 3933–3943. [Google Scholar] [CrossRef]
- Sanmartin, M.; Pateraki, I.; Chatzopoulou, F.; Kanellis, A.K. Differential expression of the ascorbate oxidase multigene family during fruit development and in response to stress. Planta 2007, 225, 873–885. [Google Scholar] [CrossRef]
- De Tullio, M.C.; Guether, M.; Balestrini, R. Ascorbate oxidase is the potential conductor of a symphony of signaling pathways. Plant Signal Behav. 2013, 8, e23213. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Ahammed, G.; Li, X.; Shi, K. Elevated CO2 improves photosynthesis under high temperature by attenuating the functional limitations to energy fluxes, electron transport and redox homeostasis in tomato leaves. Front. Plant Sci. 2018, 9, 1739. [Google Scholar] [CrossRef] [PubMed]
- Skorupa, M.; Szczepanek, J.; Yolcu, S.; Mazur, J.; Tretyn, A.; Tyburski, J. Characteristic of the Ascorbate Oxidase Gene Family in Beta vulgaris and Analysis of the Role of AAO in Response to Salinity and Drought in Beet. Int. J. Mol. Sci. 2022, 23, 12773. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gallie, D. The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell. 2004, 16, 1143–1162. [Google Scholar] [CrossRef]
- Mei, Y.; Chen, H.; Shen, W.; Shen, W.; Huang, L. Hydrogen peroxide is involved in hydrogen sulfide-induced lateral root formation in tomato seedlings. BMC Plant Biol. 2017, 17, 162. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, C.; Li, H.; Xu, Y.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Zhao, H.; Liu, B.; et al. OsJAB1 Positively Regulates Ascorbate Biosynthesis and Negatively Regulates Salt Tolerance Due to Inhibiting Early-Stage Salt-Induced ROS Accumulation in Rice. Plants 2023, 12, 3859. https://doi.org/10.3390/plants12223859
Wang J, Zhang C, Li H, Xu Y, Zhang B, Zheng F, Zhao B, Zhang H, Zhao H, Liu B, et al. OsJAB1 Positively Regulates Ascorbate Biosynthesis and Negatively Regulates Salt Tolerance Due to Inhibiting Early-Stage Salt-Induced ROS Accumulation in Rice. Plants. 2023; 12(22):3859. https://doi.org/10.3390/plants12223859
Chicago/Turabian StyleWang, Jiayi, Chuanyu Zhang, Hua Li, Yuejun Xu, Bo Zhang, Fuyu Zheng, Beiping Zhao, Haiwen Zhang, Hui Zhao, Baohai Liu, and et al. 2023. "OsJAB1 Positively Regulates Ascorbate Biosynthesis and Negatively Regulates Salt Tolerance Due to Inhibiting Early-Stage Salt-Induced ROS Accumulation in Rice" Plants 12, no. 22: 3859. https://doi.org/10.3390/plants12223859