Green Extracts and UPLC-TQS-MS/MS Profiling of Flavonoids from Mexican Oregano (Lippia graveolens) Using Natural Deep Eutectic Solvents/Ultrasound-Assisted and Supercritical Fluids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic and Flavonoid Content
2.2. Antioxidant Capacity by TEAC, FRAP, DPPH•, and ORAC Assays
2.3. Identification and Quantification of Oregano (Lippia graveolens) Flavonoid Extracts by UPLC TQS-MSMS
2.4. Total Phenolic and Total Flavonoid Content in Oregano Microcapsules
2.5. Antioxidant Capacity by ABTS•+, FRAP, and ORAC Assay in Oregano Microcapsules
2.6. Identification and Quantification of Flavonoids in Oregano (Lippia graveolens) Microcapsules
2.7. Particle Morphology
3. Materials and Methods
3.1. Chemicals and Raw Materials
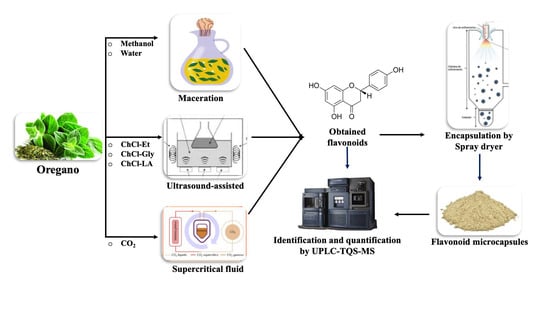
3.2. Extraction of Flavonoids from Oregano (Lippia graveolens)
3.2.1. Extraction of Flavonoids by Maceration
3.2.2. Ultrasound-Assisted Extraction of Flavonoids
3.2.3. Extraction of Flavonoids by Supercritical Fluid
3.3. Antioxidant Analysis
3.3.1. Trolox Equivalent Antioxidant Capacity (TEAC)
3.3.2. Oxygen Radical Absorbance Capacity (ORAC)
3.3.3. Antioxidant Capacity by DPPH• Assay
3.3.4. Ferric Reducing Antioxidant Power Assay (FRAP)
3.4. Total Phenolic and Flavonoid Content
3.4.1. Total Phenolic Content
3.4.2. Total Flavonoid Content
3.5. Identification and Quantification of Flavonoids by UPLC-TQS-MS/MS
3.6. Preparation of Oregano (Lippia graveolens) Microcapsules
3.7. Flavonoid Encapsulation Efficiency and Particle Morphology
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Chitala, M.D.C.; Flores-Martínez, H.; Orozco-Ávila, I.; León-Campos, C.; Suárez-Jacobo, Á.; Estarrón-EspinosaM, I. Identification and quantification of phenolic compounds from Mexican oregano (Lippia graveolens HBK) hydroethanolic extracts and evaluation of its antioxidant capacity. Molecules. 2021, 26, 702. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Nair, V.; Bang, W.Y.; Cisneros-Zevallos, L.; Heredia, J.B. Protective role of terpenes and polyphenols from three species of Oregano (Lippia graveolens, Lippia palmeri and Hedeoma patens) on the suppression of lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2016, 187, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grijalva, E.P.; Angulo-Escalante, M.A.; León-Félix, J.; Heredia, J.B. Effect of In Vitro Digestion on the Total Antioxidant Capacity and Phenolic Content of 3 Species of Oregano (Hedeoma patens, Lippia graveolens, Lippia palmeri). J. Food Sci. 2017, 82, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Picos-Salas, M.A.; Gutiérrez-Grijalva, E.P.; Valdez-Torres, B.; Angulo-Escalante, M.A.; López-Martínez, L.X.; Delgado-Vargas, F.; Heredia, J.B. Supercritical CO2 extraction of oregano (Lippia graveolens) phenolic compounds with antioxidant, α-amylase and α-glucosidase inhibitory capacity. J. Food Meas. Charact. 2021, 15, 3480–3490. [Google Scholar] [CrossRef]
- Bautista-Hernández, I.; Aguilar, C.N.; Martínez-Ávila, G.C.G.; Torres-León, C.; Ilina, A.; Flores-Gallegos, A.C.; Verma, D.K.; Chávez-González, M.L. Mexican oregano (Lippia graveolens Kunth) as source of bioactive compounds: A review. Molecules 2021, 26, 5156. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Sepúlveda, L.; Verma, D.K.; Luna-García, H.A.; Rodríguez-Durán, L.V.; Ilina, A.; Aguilar, C.N. Conventional and emerging extraction processes of flavonoids. Processes 2020, 8, 434. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Ponce-Alquicira, E. Environmentally Friendly Techniques and Their Comparison in the Extraction of Natural Antioxidants from Green Tea, Rosemary, Clove, and Oregano. Molecules 2021, 26, 1869. [Google Scholar] [CrossRef]
- Jafari, S.M.; Capanoglu, E. Extraction, processing and encapsulation of food bioactive compounds. Food Chem. 2022, 381, 132117. [Google Scholar] [CrossRef]
- Martins, A.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Fernandes, I.P.; Barreiro, F.; Ferreira, I.C.F.R. Phenolic extracts of Rubus ulmifolius Schott flowers: Characterization, microencapsulation and incorporation into yogurts as nutraceutical sources. Food Funct. 2014, 5, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, J.B.; Goltz, C.; Cavalheiro, F.B.; Toci, A.T.; Igarashi-Mafra, L.; Mafra, M.R. Deep eutectic solvents applied in the extraction and stabilization of rosemary (Rosmarinus officinalis L.) phenolic compounds. Ind. Crops Prod. 2020, 144, 112049. [Google Scholar] [CrossRef]
- Islamčević Razboršek, M.; Ivanović, M.; Krajnc, P.; Kolar, M. Choline chloride based natural deep eutectic solvents as extraction media for extracting phenolic compounds from chokeberry (Aronia melanocarpa). Molecules. 2020, 25, 1619. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, S.G. Extraction techniques of phenolic compounds from plants. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- de Almeida Pontes, P.V.; Shiwaku, I.A.; Maximo, G.J.; Caldas Batista, E.A. Choline chloride-based deep eutectic solvents as potential solvent for extraction of phenolic compounds from olive leaves: Extraction optimization and solvent characterization. Food Chem. 2021, 352, 129346. [Google Scholar] [CrossRef] [PubMed]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Natural antioxidants of plant origin. Adv. Food Nutr. Res. 2019, 90, 1–81. [Google Scholar] [CrossRef] [PubMed]
- Alsaud, N.; Shahbaz, K.; Farid, M. Application of deep eutectic solvents in the extraction of polyphenolic antioxidants from New Zealand Manuka leaves (Leptospermum scoparium): Optimization and antioxidant activity. J. Mol. Liq. 2021, 337, 116385. [Google Scholar] [CrossRef]
- Zúñiga-Núñez, D.; Barrias, P.; Cárdenas-Jirón, G.; Ureta-Zañartu, M.S.; Lopez-Alarcón, C.; Morán-Vieyra, F.E.; Borsarelli, C.D.; Alarcon, E.I.; Aspée, A. Atypical antioxidant activity of non-phenolic amino-coumarins. RSC Adv. 2018, 8, 1927–1933. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods. 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Garcia-Carrasco, M.; Picos-Corrales, L.A.; Gutiérrez-Grijalva, E.P.; Angulo-Escalante, M.A.; Licea-Claverie, A.; Heredia, J.B. Loading and release of phenolic compounds present in Mexican oregano (Lippia graveolens) in different chitosan bio-polymeric cationic matrixes. Polymers 2022, 14, 3609. [Google Scholar] [CrossRef]
- Arias, J.; Mejía, J.; Córdoba, Y.; Martínez, J.R.; Stashenko, E.; del Valle, J.M. Optimization of flavonoids extraction from Lippia graveolens and Lippia origanoides chemotypes with ethanol-modified supercritical CO2 after steam distillation. Ind. Crops Prod. 2020, 146, 112170. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Hernández-Sánchez, L.Y.; Muñoz Ocotero, V.; Dorazco-González, A.; Guevara Fefer, P.; Aguirre-Hernández, E. Pharmacological evaluation of the anxiolytic-like effects of Lippia graveolens and bioactive compounds. Pharm. Biol. 2017, 55, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Táborský, J.; Sus, J.; Lachman, J.; Šebková, B.; Adamcová, A.; Šatínský, D. Dynamics of phloridzin and related compounds in four cultivars of apple trees during the vegetation period. Molecules 2021, 26, 3816. [Google Scholar] [CrossRef]
- Zielinska, D.; Laparra-Llopis, J.M.; Zielinski, H.; Szawara-Nowak, D.; Giménez-Bastida, J.A. Role of apple phytochemicals, phloretin and phloridzin, in modulating processes related to intestinal inflammation. Nutrients 2019, 11, 1173. [Google Scholar] [CrossRef]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The bioavailability, extraction, biosynthesis and distribution of natural dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic properties of naringenin: A citrus fruit polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Palombo, R.; Savini, I.; Avigliano, L.; Madonna, S.; Cavani, A.; Albanesi, C.; Mauriello, A.; Melino, G.; Terrinoni, A. Luteolin-7-glucoside inhibits IL-22/STAT3 pathway, reducing proliferation, acanthosis, and inflammation in keratinocytes and in mouse psoriatic model. Cell Death Dis. 2016, 7, e2344. [Google Scholar] [CrossRef]
- Bernal-Millán, M.J.; Gutiérrez-Grijalva, E.P.; Contreras-Angulo, L.; Muy-Rangel, M.D.; López-Martínez, L.X.; Heredia, J.B. Spray-dried microencapsulation of oregano (Lippia graveolens) polyphenols with maltodextrin enhances their stability during in vitro digestion. J. Chem. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of extracts of bioactive compounds obtained from acerola (Malpighia emarginata DC) pulp and residue by spray and freeze drying: Chemical, morphological and chemometric characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef]
- Peng, Z.; Li, J.; Guan, Y.; Zhao, G. Effect of carriers on physicochemical properties, antioxidant activities and biological components of spray-dried purple sweet potato flours. Lebenson Wiss Technol. 2013, 51, 348–355. [Google Scholar] [CrossRef]
- Ruiz-Canizales, J.; Heredia, J.B.; Domínguez-Avila, J.A.; Madera-Santana, T.J.; Villegas-Ochoa, M.A.; Robles-Sánchez, R.M.; González-Aguilar, G.A. Microencapsulation of blue maize (Zea mays L.) polyphenols in two matrices: Their stability during storage and in vitro digestion release. J. Food Meas. Charact. 2019, 13, 892–900. [Google Scholar] [CrossRef]
- Desai, N.M.; Haware, D.J.; Basavaraj, K.; Murthy, P.S. Microencapsulation of antioxidant phenolic compounds from green coffee. Prep. Biochem. Biotechnol. 2019, 49, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Batista, V.S.F.; Nunes, G.L.; Viegas, G.I.; Lucas, B.N.; Bochi, V.C.; Emanuelli, T.; Barin, J.S.; Ragagnin de menezes, C.; Severo da Rosa, C. Extraction, characterization and microencapsulation of isoflavones from soybean molasses. Cienc. Rural. 2020, 50, 1–7. [Google Scholar] [CrossRef]
- Ydjedd, S.; Bouriche, S.; López-Nicolás, R.; Sánchez-Moya, T.; Frontela-Saseta, C.; Ros-Berruezo, G.; Rezgui, F.; Louaileche, H.; Kati, D.E. Effect of in vitro gastrointestinal digestion on encapsulated and nonencapsulated phenolic compounds of carob (Ceratonia siliqua L.) pulp extracts and their antioxidant capacity. J. Agric. Food Chem. 2017, 65, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Omidvar, V.; Jaafar, H.Z.E. Polyphenolic content and their antioxidant activity in leaf extract of sweet potato (Ipomoea batatas). J. Med. Plant Res. 2012, 6, 2971–2976. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]


| Sample/Extracts | Total Phenolic (mg GAE/g) | Total Flavonoids (mg QE/g) |
|---|---|---|
| Methanol | 66.2 ± 3.4 b | 26.9 ± 1.8 bc |
| Water | 27.9 ± 3.4 c | 19.2 ± 0.3 c |
| ChCl-Et | 100.1 ± 7.5 ab | 39.5 ± 3.3 a |
| ChCl-Gly | 123.6 ± 9.8 a | 39.4 ± 2.9 a |
| ChCl-LA | 126.1 ± 6.0 a | 37.3 ± 3.0 ab |
| Supercritical CO2 | 17.5 ± 1.3 c | 5.2 ± 0.8 d |
| Compounds | Compound Type | Method/Solvent | |||||
|---|---|---|---|---|---|---|---|
| Maceration/ Methanol | Maceration/ Water | Ultrasound-Assisted/ ChCl-Et | Ultrasound-Assisted/ ChCl-Gly | Ultrasound-Assisted/ ChCl-LA | Supercritical/ CO2-Ethanol | ||
| Quercetin | Flavonol | 290.04 ± 2.88 b | 8.92 ± 0.91 c | 3.54 ± 0.85 d | 9.93 ± 3.16 c | 2.60 ± 1.61 d | 432.69 ± 0.14 a |
| Vitexin | Flavone | ND b | ND b | ND b | 1.24 ± 0.63 a | 1.30 ± 0.09 a | ND |
| Apigenin | Flavone | 15.58 ± 3.05 b | 1.03 ± 0.25 c | ND c | ND c | ND c | 22.22 ± 3.31 a |
| Quercitrin | Flavonol | 12.57 ± 2.03 ab | 4.59 ± 0.70 c | 13.86 ± 1.45 a | 10.05 ± 1.33 b | 4.66 ± 0.88 c | ND d |
| Luteolin | Flavone | 181.28 ± 36.69 | 26.79 ± 3.73 | ND | 3.58 ± 0.80 | ND | 7.63 ± 1.06 |
| L7G | Flavone | 1247.84 ± 68.73 b | 489.54 ± 15.09 e | 1334.14 ± 6.78 a | 664.22 ± 21.29 d | 765.73 ± 17.83 c | 1.15 ± 0.26 f |
| Naringenin | Flavanone | 3505.74 ± 163.60 a | 452.48 ± 22.21 b | 430.31 ± 3.46 b | 286.85 ± 72.07 b | 102.33 ± 21.13 c | 3513.78 ± 113.69 a |
| Naringin | Flavanone | 1.00 ± 0.10 b | ND b | 1.50 ± 0.36 b | 1.49 ± 0.24 b | 4.77 ± 1.42 a | ND b |
| Genistein | Isoflavone | 15.44 ± 1.91 b | ND c | ND c | ND c | ND c | 24.03 ± 3.44 a |
| Rutin | Flavonol | 2.37 ± 0.92 de | 5.25 ± 0.09 d | 36.95 ± 2.61 a | 30.01 ± 1.19 b | 11.24 ± 3.48 c | ND e |
| Hesperidin | Flavanone | 25.56 ± 3.58 b | 11.70 ± 2.64 bc | 101.65 ± 2.94 a | 85.81 ± 2.65 a | 81.56 ± 31.56 a | ND c |
| Kaempferol | Flavonol | 12.11 ± 2.40 ab | 9.83 ± 0.27 b | ND d | 2.56 ± 0.04 c | ND d | 14.22 ± 1.5 a |
| Phloretin | Dihydrochalcone | 6.26 ± 0.17 b | 7.50 ± 0.23 b | ND c | 1.61 ± 0.32 c | ND c | 37.78 ± 2.46 a |
| Phloridzin | Dihydrochalcone | 4068.91 ± 50.59 a | 1306.56 ± 49.05 d | 3049.26 ± 80.93 b | 1773.93 ± 23 c | 1177.2 ± 100.32 e | 73.61 ± 8.26 f |
| Sample | TPC (mg GAE/g Sample) | TFC (mg QE/g Sample) | ABTS•+ (μmol TE/g Sample) | FRAP (μmol TE/g Sample) | ORAC (μmol TE/g Sample) |
|---|---|---|---|---|---|
| Microcapsules | 17.3 ± 2.1 | 5.0 ± 0.3 | 241.9 ± 4.7 | 178.6 ± 2.6 | 637.0 ± 4.6 |
| Non-microencapsulated extract | 83.7 ± 5.9 | 25.1 ± 2.9 | 1198.6 ± 55.5 | 992.6 ± 20.8 | 4153.8 ± 133.1 |
| Compounds | Compound Type | Microcapsules (μg/g) | Non-Microencapsulated Extract (μg/g) |
|---|---|---|---|
| Quercetin | Flavonol | 329.43 ± 28.73 | 1916.0 ± 164.61 |
| Vitexin | Flavone | ND | ND |
| Apigenin | Flavone | 22.40 ± 2.21 | 53.23 ± 7.34 |
| Quercitrin | Flavonol | ND | ND |
| Luteolin | Flavone | 17.29 ± 0.58 | 64.24 ± 1.18 |
| Luteolin-7-glucoside | Flavone | 67.48 ± 1.74 | 111.63 ± 8.47 |
| Naringenin | Flavanone | 1181.35 ± 104.23 | 3840.11 ± 199.92 |
| Naringin | Flavanone | ND | ND |
| Genistein | Isoflavone | 15.00 ± 2.70 | 80.25 ± 0.97 |
| Rutin | Flavonol | 3.04 ± 0.15 | 8.54 ± 0.73 |
| Hesperidin | Flavanone | 3.49 ± 0.58 | 10.09 ± 0.46 |
| Kaempferol | Flavonol | 17.33 ± 1.66 | 82.11 ± 4.54 |
| Phloretin | Dihydrochalcone | ND | ND |
| Phloridzin | Dihydrochalcone | 677.92 ± 6.72 | 1202.08 ± 35.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal-Millán, M.d.J.; Carrasco-Portugal, M.d.C.; Heredia, J.B.; Bastidas-Bastidas, P.d.J.; Gutiérrez-Grijalva, E.P.; León-Félix, J.; Angulo-Escalante, M.Á. Green Extracts and UPLC-TQS-MS/MS Profiling of Flavonoids from Mexican Oregano (Lippia graveolens) Using Natural Deep Eutectic Solvents/Ultrasound-Assisted and Supercritical Fluids. Plants 2023, 12, 1692. https://doi.org/10.3390/plants12081692
Bernal-Millán MdJ, Carrasco-Portugal MdC, Heredia JB, Bastidas-Bastidas PdJ, Gutiérrez-Grijalva EP, León-Félix J, Angulo-Escalante MÁ. Green Extracts and UPLC-TQS-MS/MS Profiling of Flavonoids from Mexican Oregano (Lippia graveolens) Using Natural Deep Eutectic Solvents/Ultrasound-Assisted and Supercritical Fluids. Plants. 2023; 12(8):1692. https://doi.org/10.3390/plants12081692
Chicago/Turabian StyleBernal-Millán, Manuel de Jesús, Miriam del Carmen Carrasco-Portugal, J. Basilio Heredia, Pedro de Jesús Bastidas-Bastidas, Erick Paul Gutiérrez-Grijalva, Josefina León-Félix, and Miguel Ángel Angulo-Escalante. 2023. "Green Extracts and UPLC-TQS-MS/MS Profiling of Flavonoids from Mexican Oregano (Lippia graveolens) Using Natural Deep Eutectic Solvents/Ultrasound-Assisted and Supercritical Fluids" Plants 12, no. 8: 1692. https://doi.org/10.3390/plants12081692