1. Introduction
Skin is the largest and most complex organ in the human body. The skin serves as a barrier between the body and the outside environment, and it serves a variety of functions [
1]. It has a significant cosmetic role in addition to protecting the body from water loss and microbial infection [
2]. In addition, it works to support other body parts, such as the immune, nervous and endocrine systems [
1]. The look of youth and beauty may have a positive impact on people’s social behavior and human life [
1,
2]. Hence, the impairment of skin structure and functions that occur as we age might have a severe impact on our health and well-being [
2,
3]. Thinness, dryness, lack of elasticity, rough texture, wrinkles, and dark pigments are all common characteristics of older skin [
4]. Many researchers are currently working on generating potential anti-aging drugs or chemicals, particularly those derived from natural sources for skin aging treatment.
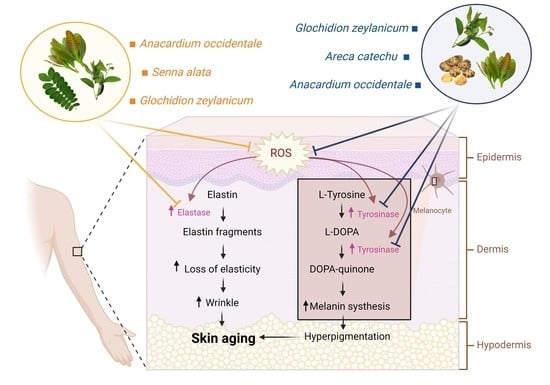
In general, human skin ages in two ways: internally (as a result of chronological aging) and extrinsically (as a result of environmental variables influenced by environmental factors) [
5]. Extensive evidence suggests that oxidative stress, through the formation of reactive oxygen species (ROS), plays a fundamental role in both intrinsic and extrinsic skin aging [
6]. ROS causes oxidative damage to skin cells by damaging essential macromolecules such as nucleic acids, enzymatic proteins, and membrane lipids, resulting in cellular malfunction and cell death [
7]. Oxidative stress also contributes to the degradation of the extracellular matrix (ECM) by suppressing ECM component synthesis (e.g., elastin) and activating ECM degrading enzymes (e.g., elastase), which results in loss of skin elasticity [
2,
8,
9]. Moreover, ROS can cause irregular or dark colors in the skin by inducing the production of an α-melanocyte-stimulating hormone (α-MSH) in keratinocytes, thereby triggering the activation of the tyrosinase enzyme and promoting melanin synthesis in melanocytes [
10,
11]. Therefore, scavenging ROS and inhibiting elastase and tyrosinase activities could be useful in the treatment or even prevention of skin aging.
Natural products are currently receiving much interest as potential alternative medicines for treating a number of diseases as well as aging and age-associated declines [
8,
12,
13]. Tropical plants could be of interest to explore their potential in skin aging treatments. As reported previously, several Andean and Himalayan plants have been regarded as sources of compounds with potential use as anti-aging ingredients [
14,
15]. Thailand is a known place for cultivating a wide variety of tropical plants, many of which have not been studied extensively. The goal of this research was to find potential natural sources for developing novel treatments against skin aging. The extracts of 16 Thai medicinal plant species were studied in vitro for their properties related to anti-skin aging, including total phenolic and flavonoid contents, free radical scavenging, anti-elastase, and anti-tyrosinase activities. We also further performed correlation analysis and an in silico molecular docking approach to reveal the promising phytochemical compounds in the three most effective plants with strong inhibition against elastase or tyrosinase enzyme.
3. Discussion
Skin aging is a naturally occurring process in all human beings. However, many lifestyles and environmental factors can also accelerate this process leading to prematurely aged skin [
19]. ROS is well known as an important pathogenic factor in the aging process of the skin. The accumulation of ROS can upregulate the expression of both elastase and tyrosinase enzymes, which subsequently leads to wrinkle formation, lack of elasticity, and hyperpigmentation [
20,
21,
22]. All of these are common characteristics of skin aging [
4]. Here, our study investigated the antioxidant, anti-elastase, and anti-tyrosinase properties of plant extracts from 16 Thai plant species and revealed promising natural compounds for the potential development of novel treatments against skin aging.
Elastase is a protease enzyme that is primarily responsible for the degradation of elastin, an important protein found in the ECM. Elastin is vital for giving elasticity to the skin due to its elastic recoil properties [
13]. Therefore, the inhibition of elastase activity can be helpful in preventing skin loss of elasticity and wrinkles [
23]. Our result found that
A. occidentale was the most potent elastase inhibitor, followed by
G. zeylanicum and
S. alata, in rank order of IC
50 values. Furthermore, the docking results revealed that five compounds derived from these three plants have lower binding energies than EGCG (positive control) and FRW (original inhibitor), wherein the compounds displaying the lower binding energy were considered to have better inhibition (
Table S4) [
24]. Those compounds are flavonoids, which include a tetramer of proanthocyanidin [
25], amentoflavone [
25], rutin [
26,
27], agathisflavone [
26] from
A. occidentale, and kaempferol 3-O-gentiobioside from
S. alata [
28] (
Table S3). The results suggested all five compounds to be responsible for anti-elastase activity as well as can be regarded as promising candidates for the development of anti-skin aging. However, we found that none of the compounds from
G. zeylanicum showed strong binding affinity as compared to the control ligands, although its extract showed the second most activity by in vitro assay. This may be due to the synergistic effect of compounds in the mixture rather than the individual effect of each phytochemical component.
In addition to elastase, melanin is considered another important target for skin-aging treatment. Melanin is a major component of the skin, hair, and eye color synthesized by melanogenesis within the melanocyte. However, overproduction of melanin may cause skin disorders, including freckles, melasma, age spots, and hyperpigmentation, leading to a premature aging appearance [
12]. In melanogenesis, tyrosinase is the critical enzyme in the rate-limiting step. Therefore, the downregulation of tyrosinase activity can lead to reduced melanin production [
29]. Our results showed that
G. zeylanicum was the most potent tyrosinase inhibitor, followed by
A. catechu and
A. occidentale, according to IC
50 values. Surprisingly, most of the compounds (47 of 48 compounds) in these three plants showed lower binding energy against tyrosinase than KA (positive control) and tropolone (original inhibitor) (
Table S5). Among the 47 compounds, 16 were derived from
G. zeylanicum, 12 were derived from
A. catechu, and 29 were derived from
A. occidentale, of which 10 of them can be found in more than one plant (
Table S3). However, in contrast to the binding of the compound with elastase, the identified potential compounds against tyrosinase are from a variety of phytochemical classes. Regarding the rank of binding energies towards tyrosinase, the compounds that are considered the best five inhibitors are o-Coumaric acid [
30] (phenolic), tetramer of proanthocyanidin [
25] (flavonoid), caffeic acid [
30] (phenolic), ferulic acid [
30] (phenolic), and arecatannin A1 [
31] (tannin), in the increasing order of scores (
Table S5). The results obtained from in vitro screening and docking analysis in this study have confirmed the anti-skin-aging properties of four Thai medicinal plants and suggested their potential derived compounds that could be responsible for the observed activities. Further studies on fractionation, as well as the identification and isolation of bioactive compounds in these promising plant extracts, are critically required to prove the presence of our proposed molecules that could subsequently be developed for the treatment of aging skin [
14].
A. catechu,
A. occidentale,
G. zeylanicum, and
S. alata are plants used in traditional medicine and found in the tropical zone of Southeast Asia, including Thailand [
32,
33,
34,
35,
36]. Notably, these plants were demonstrated for several antioxidant-related activities.
A. catechu fruit is a popular chewable item with betel leaves, which is intoxicating and slightly addictive [
36]. It is used for the treatment of burn wounds and skin ulcers and acts as an astringent [
35,
37]. It has been reported for potent antioxidant and anti-inflammatory effects against oxidative stress-induced liver injury in rats [
38].
A. occidentale and
G. zeylanicum, belonging to Southern Thailand, are used as food and local medicinal plant [
32,
33].
A. occidentale leaves are used to treat skin rashes, itching, ulcers, and fever, whereas
G. zeylanicum leaves are used to treat rheumatoid arthritis, influenza, dysentery, and dyspepsia [
32,
33,
39]. The leaf extracts of
A. occidentale and
G. zeylanicum exhibited neuroprotective effects against glutamate and H
2O
2-induced oxidative damage [
32,
40]. Moreover, crude extract from the leaves of both plants exerted antioxidative stress and anti-aging properties in the nematode
Caenorhabditis elegans [
33,
41,
42]. Leaves of
S. alata are used for the treatment of skin rashes, mycosis, and dermatitis [
34]. Leaf extract of this plant was able to increase both enzymatic and nonenzymatic antioxidant systems and prevent the liver and renal tissues from damage caused by oxidative stress during diabetes in a rat model [
43].
In agreement with previous reports, our findings reveal that
A. catechu,
A. occidentale,
G. zeylanicum, and
S. alata exhibit antioxidant potential, apart from the activities toward skin aging-related enzymes. We found that these plants are rich in total phenolics and flavonoids with high antioxidant capacities towards DPPH and ABTS radicals, except for
S. alata, which showed only a moderate level in both amounts and activities. Phenolics and flavonoids are two well-known classes of plant secondary metabolites that are majorly responsible for antioxidant activity [
44,
45]. This was consistent with our results that total phenolic and flavonoid contents demonstrated a significant positive correlation with free radical scavenging activities (
Figure S1). Interestingly, the correlation analysis also revealed that the contents of total phenolic and flavonoid compounds in this studied plant extracts were positively correlated with both elastase and tyrosinase inhibition. However, the correlation strength was found to be higher with phenolics than with flavonoids. Free radical scavenging activities were shown to have a high correlation to the enzyme-inhibitory activities of the extracts. These results suggested that high phenolic content and antioxidant activity may lead to strong inhibition of elastase and tyrosinase enzymes. However, the possibility of protein-polyphenol interactions should also be a concern. Some polyphenols could directly cause enzyme precipitation via their ability to bind with proline-rich proteins, resulting in hydrogen-bond formation with the enzyme and thereby leading to non-selective inhibition [
46].
4. Materials and Methods
4.1. Chemicals and Reagents
Folin–Ciocalteu’s phenol reagent, aluminum chloride (AlCl3), dimethyl sulfoxide (DMSO), sodium acetate (NaOAc), quercetin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), L-ascorbic acid, potassium persulfate (K2S2O8), elastase from porcine pancreas, epigallocatechin gallate (EGCG), N-succinyl-Ala-Ala-Ala-p-nitroanilide (SANA), tyrosinase from mushroom, kojic acid (KA), and 3,4-dihydroxy-L-phenylalanine (L-DOPA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium carbonate (Na2CO3) was purchased from Merck (Darmstadt, Germany). Gallic acid was purchased from TCI America (Portland, OR, USA). Dipotassium phosphate (K2HPO4) and monobasic potassium phosphate (KH2PO4) were purchased from HiMedia (Mumbai, India). Tris base was purchased from Vivantis Technologies (Shah Alam, Malaysia). Ethanol and methanol were purchased from RCI Labscan (Bangkok, Thailand). All chemicals and reagents were analytical grades.
4.2. Plant Materials and Extraction
The plants in this study were collected locally from gardens or purchased from local markets as appropriate.
Table 1 provides the scientific name, part used, and source of each plant. These plants were botanically authenticated, and their voucher specimens were deposited in the herbarium of Kasin Suvatabhandhu, Department of Botany, Faculty of Science, Chulalongkorn University, Bangkok, Thailand, or identified by a botanist. The plant materials were washed, dried at 65 °C, and ground finely in a mechanical grinder. The extraction of the dried plant (40 g) was carried out by Soxhlet extraction or maceration method using 400 mL of ethanol or methanol. The extracts were filtered and evaporated to dryness under a vacuum. Then, the dried residues were dissolved in DMSO as a 100 mg/mL stock solution and stored at −20 °C for further study.
4.3. Determination of Total Phenolic Content
The total phenolic content was performed using the Folin–Ciocalteu method [
47]. Briefly, 50 µL of extracts at 1 mg/mL in deionized water was mixed with 50 µL of 10% (
w/
v) Folin–Ciocalteu’s phenol reagent in a 96-well plate and incubated in the dark at room temperature (RT) for 20 min. After the incubation, 50 µL of 7.5% (
w/
v) Na
2CO
3 was added to the mixture and incubated for a further 20 min. The absorbance was measured with a microplate reader at 760 nm. The total phenolic content was calculated from a standard calibration curve using gallic acid from 1.56 to 100 µg/mL, and the results are shown as mg of gallic acid equivalent (GAE) per g dry weight extract.
4.4. Determination of Total Flavonoid Content
The total flavonoid content was performed using aluminum chloride (AlCl
3) [
47]. Briefly, 50 µL of extracts at 1 mg/mL in deionized water was made up to 200 µL with 95% ethanol, and then 10 µL of 10% AlCl
3 and 10 µL of 1 M NaOAc were added to a 96-well plate. The plate was incubated in the dark at RT for 40 min, and absorbance was measured with a microplate reader at 415 nm. The total flavonoid content was calculated from a standard calibration curve using quercetin from 1.56 to 100 µg/mL, and the results showed as mg of quercetin equivalent (QE) per g dry weight extract.
4.5. Determination of DPPH Radical Scavenging Activity
DPPH radical scavenging activity assay was performed as described previously [
47]. The DPPH
• working reagent was prepared by DPPH dissolved in absolute ethanol. Briefly, 180 µL of DPPH
• working solution was mixed with 20 µL of extracts in a 96-well plate and was incubated in the dark at RT for 15 min, and absorbance was measured with a microplate reader at 517 nm. Ascorbic acid from 1.56 to 100 μg/mL served as a standard. The radical scavenging activity was calculated as the percent inhibition of free radicals using the following equation:
Percentages of DPPH scavenging activity of each plant extract were compared with those of ascorbic acid. The results were expressed as mg of vitamin C equivalent antioxidant capacity (VCEAC) per g dry weight extract. The IC50 (half-maximal inhibitory concentration) was determined from the graph of percent inhibition against the concentration of each extract.
4.6. Determination of ABTS Radical Scavenging Activity
ABTS radical scavenging activity assay was performed as described previously [
47]. The ABTS
•+ working reagent was prepared by mixing 7 mM ABTS
• and 2.45 mM K
2S
2O
8 at a ratio of 1:1, and the mixture had to remain for 16–18 h in the dark at RT. The ABTS
•+ working solution was diluted with absolute ethanol for the absorbance to reach between 0.7 and 0.8 at 734 nm. Briefly, 180 µL of ABTS
•+ working solution was mixed with 20 µL of extracts in a 96-well plate and was incubated in the dark at RT for 30 min, and absorbance was measured with a microplate reader at 734 nm. Ascorbic acid from 1.56 to 100 μg/mL served as a standard. The radical scavenging activity was calculated as the percent inhibition of free radicals using the Equation (1).
Percentages of ABTS scavenging activity of each plant extract were compared with those of ascorbic acid. The results expressed as mg of vitamin C equivalent antioxidant capacity (VCEAC) per g dry weight extract. The IC50 was determined from the graph of percent inhibition against the concentration of each extract.
4.7. Determination of Anti-Elastase Activity
The anti-elastase activity was evaluated by the elastase inhibition assay using the modified protocol [
13,
48,
49]. Briefly, 20 µL of extracts, 10 µL of 0.4 U/mL pancreatic porcine elastase (PPE), and 140 µL of 0.1 M Tris-HCL buffer at pH 8.0 were added in 96-well plate and pre-incubated at RT for 20 min. After incubation, 30 µL of 2 mM SANA was added to the reaction mixture and further incubated for 30 min at RT. The absorbance was measured with a microplate reader at 734 nm. EGCG was used to serve as a positive control for inhibition. The negative control contained 100% DMSO instead of the extracts. The percent inhibition of elastase activity was calculated using the equation (1). The IC
50 was determined from the graph of percent elastase inhibition against a concentration of each extract.
4.8. Determination of Anti-Tyrosinase Activity
The anti-tyrosinase activity was performed using the dopachrome method with some modifications [
8]. Briefly, 20 µL of extracts, 20 µL of 200 U/mL mushroom tyrosinase, and 140 µL of 0.1 M phosphate buffer at pH 6.8 were added in 96-well plates and pre-incubated in RT for 20 min. After incubation, 40 µL of 2.5 mM L-DOPA was added to the reaction mixture and further incubated for 20 min at RT. The absorbance was read with a microplate reader at 492 nm. KA was used to serve as a positive control for inhibition. The negative control contained 100% DMSO instead of the extracts. The percent inhibition of tyrosinase activity was calculated using the equation (1). The IC
50 was determined from the graph of percent tyrosinase inhibition against a concentration of each extract.
4.9. Molecular Docking
4.9.1. Ligand Preparation
A list of phytochemical compounds from the three most effective plants with strong inhibition against elastase or tyrosinase was selected from the published literature [
25,
26,
27,
28,
30,
31,
32,
42,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61] (
Table S3). All chemical structures of the compounds were generated from the IUPAC name using BIOVIA Draw 2019 (BIOVIA, San Diego, CA, USA). Then, the compounds were cleaned geometry and saved the file to format pdb using Discovery Studio Visualizer (BIOVIA, San Diego, CA, USA). These files were converted to format pdbqt using AutoDockTools-1.5.6 software (The Scripps Research Institute, San Diego, CA, USA).
4.9.2. Protein Preparation
The X-ray crystallographic structures of elastase (PDB ID: 3HGP) [
62] and tyrosinase (PDB ID: 2Y9X) [
63] were obtained from RCSB Protein Data Bank. Before the docking study, using Discovery Studio Visualizer, water molecules and the original inhibitor were removed from the protein structure, excluding Cu
2+ in structures of tyrosinase. These protein structures were prepared using the prepared protein setup in AutoDockTools-1.5.6 software. All missing hydrogens and Kollman charges were added to the protein structure and saved in the file to format pdbqt for docking study.
4.9.3. Molecular Docking
Molecular docking studies were performed using the default protocol in AutoDockTools-1.5.6 software. Grid sites were set with a spacing of 0.375 Å. The x–y–z dimensions were set to 40 × 40 × 40 points for elastase and 60 × 60 × 60 points for tyrosinase. Grid box of the x, y, and z centers were 12.58, 9.36, and 2.251 for elastase and −10.044, −28.706, and −43.443 for tyrosinase. The docking study was performed using the Lamarckian Genetic Algorithm (GA) with default parameters, and docking results were analyzed using AutoDockTools-1.5.6 software and Discovery Studio Visualizer.
4.10. Statistical Analysis
All experiments were performed in at least triplicate, and the results were represented as the mean ± standard deviation (SD). The IC50 values were analyzed using SigmaPlot version 12.0 software. Correlation between different variables was expressed as Pearson’s correlation coefficients (r). The correlation was determined by using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA), and the results were considered statistically significant when p was less than 0.05 (p < 0.05).