“Live-Autoradiography” Technique Reveals Genetic Variation in the Rate of Fe Uptake by Barley Cultivars
Abstract
:1. Introduction
2. Results
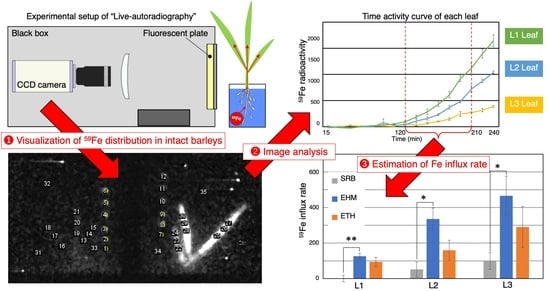
2.1. Improvements in Live-Autoradiography for Phenotyping of Fe Uptake
2.2. Preparation of Barley Plants with Chlorosis Symptoms and Decreased Fe Concentration in the Leaves for 59Fe Imaging
2.3. The Influx Rate of a Trace Amount of Fe Was Not Always Higher in Tolerant Cultivars
2.4. Downregulation Confirmation of the SUF Pathway and Accumulation of Reaction-Center Proteins
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Fe Determination
4.3. Live-Autoradiography and 59Fe Imaging
4.4. Quantitative Polymerase Chain Reaction Analysis
4.5. Western Blot Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hubeau, M.; Steppe, K. Plant-PET scan: In vivo mapping of xylem and phloem functioning. Trends Plant Sci. 2015, 20, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, S.; Menzel, M.I.; van Dusschoten, D.; Roeb, G.W.; Bühler, J.; Minwuyelet, S.; Blümler, P.; Temperton, V.M.; Hombach, T.; Streun, M.; et al. Combined MRI–PET dissects dynamic changes in plant structures and functions. Plant J. 2009, 59, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Streun, M.; Beer, S.; Daemen, J.; Dorscheid, R.; Erven, A.; Jokhovets, L.; Meessen, L.; Mekala, N.B.; Mülhens, O.; Nöldgen, H.; et al. PhenoPET: A dedicated PET scanner for plant research based on digital SiPMs (DPCs). In Proceedings of the 2014 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Seattle, WA, USA, 8–15 November 2014; Institute of Electrical and Electronics Engineers: Piscataway, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Fujimaki, S.; Maruyama, T.; Suzui, N.; Kawachi, N.; Miwa, E.; Higuchi, K. Base to tip and long-distance transport of sodium in the root of common reed [Phragmites australis (Cav.) Trin. ex Steud.] at steady state under constant high-salt conditions. Plant Cell Physiol. 2015, 56, 943–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruwanpathirana, G.P.; Plett, D.C.; Williams, R.C.; Davey, C.E.; Johnston, L.A.; Kronzucker, H.J. Continuous monitoring of plant sodium transport dynamics using clinical PET. Plant Methods 2021, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Brezovcsik, K.; Veres, S.; Molnár, J.; Fenyvesi, A.; Szűcs, Z. Comparison of manganese uptake and transport of maize seedlings by mini-PET camera. Appl. Radiat. Isot. 2020, 160, 109127. [Google Scholar] [CrossRef]
- Kroh, G.E.; Pilon, M. Regulation of iron homeostasis and use in chloroplasts. Int. J. Mol. Sci. 2020, 21, 3395. [Google Scholar] [CrossRef]
- George, E.; Horst, W.J.; Neumann, E. 17.5.1.1 Iron. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Clemens, S. Metal ligands in micronutrient acquisition and homeostasis. Plant Cell Environ. 2019, 42, 2902–2912. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Nozoye, T.; Nishizawa, N.K. Iron transport and its regulation in plants. Free Radic. Biol. Med. 2019, 133, 11–20. [Google Scholar] [CrossRef]
- Saito, A.; Shinjo, S.; Ito, D.; Doi, Y.; Sato, A.; Wakabayashi, Y.; Honda, J.; Arai, Y.; Maeda, T.; Ohyama, T.; et al. Enhancement of photosynthetic iron-use efficiency is an important trait of Hordeum vulgare for adaptation of photosystems to iron deficiency. Plants 2021, 10, 234. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Nakanishi, H.; Uchida, H.; Watanabe, S.; Matsuhashi, S.; Mori, S.; Nishizawa, N.K. 52Fe Translocation in barley as monitored by a Positron-Emitting Tracer Imaging System (PETIS): Evidence for the direct translocation of Fe from roots to young Leaves via phloem. Plant Cell Physiol. 2009, 50, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, T.M.; Yamawaki, M.; Kannno, S.; Nihei, N.; Masuda, S.; Tanoi, K. Real-time imaging of ion uptake from root to above-ground part of the plant using conventional beta-ray emitters. J. Radioanal. Nucl. Chem. 2009, 282, 265–269. [Google Scholar] [CrossRef]
- Sugita, R.; Kobayashi, N.I.; Hirose, A.; Saito, T.; Iwata, R.; Tanoi, K.; Nakanishi, T.M. Visualization of uptake of mineral elements and the dynamics of photosynthates in arabidopsis by a newly developed real-time radioisotope imaging system (RRIS). Plant Cell Physiol. 2016, 57, 743–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurita, K.; Sakai, T.; Suzui, N.; Yin, Y.G.; Sugita, R.; Kobayashi, N.I.; Tanoi, K.; Kawachi, N. Autoradiography system with phosphor powder (ZnS:Ag) for imaging radioisotope dynamics in a living plant. Jpn. J. Appl. Phys. 2021, 60, 116501. [Google Scholar] [CrossRef]
- Bughio, N.; Takahashi, M.; Yoshimura, E.; Nishizawa, N.K.; Mori, S. Light-dependent iron transport into isolated barley chloroplasts. Plant Cell Physiol. 1997, 38, 101–105. [Google Scholar] [CrossRef]
- Kobayashi, T. Understanding the complexity of iron sensing and signaling cascades in plants. Plant Cell Physiol. 2019, 60, 1440–1446. [Google Scholar] [CrossRef]
- Braymer, J.J.; Freibert, S.A.; Rakwalska-Bange, M.; Lill, R. Mechanistic concepts of iron–sulfur protein biogenesis in biology. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118863. [Google Scholar] [CrossRef]
- Hantzis, L.J.; Kroh, G.E.; Jahn, C.E.; Cantrell, M.; Peers, G.; Pilon, M.; Ravet, K. A program for iron economy during deficiency targets specific Fe proteins. Plant Physiol. 2018, 176, 596–610. [Google Scholar] [CrossRef] [Green Version]
- Kroh, G.E.; Pilon, M. Iron deficiency and the loss of chloroplast iron–sulfur cluster assembly trigger distinct transcriptome changes in Arabidopsis rosettes. Metallomics 2020, 12, 1748–1764. [Google Scholar] [CrossRef]
- Liang, X.; Qin, L.; Liu, P.; Wang, M.; Ye, H. Genes for iron–sulphur cluster assembly are targets of abiotic stress in rice, Oryza sativa. Plant Cell Environ. 2014, 37, 780–794. [Google Scholar] [CrossRef]
- Georg, J.; Kostova, G.; Vuorijoki, L.; Schön, V.; Kadowaki, T.; Huokko, T.; Baumgartner, D.; Müller, M.; Klähn, S.; Allahverdiyeva, Y.; et al. Acclimation of oxygenic photosynthesis to iron starvation is controlled by the sRNA IsaR1. Curr. Biol. 2017, 27, 1425–1436. [Google Scholar] [CrossRef]
- Schmidt, S.B.; George, T.S.; Brown, L.K.; Booth, A.; Wishart, J.; Hedley, P.E.; Martin, P.; Russell, J.; Husted, S. Ancient barley landraces adapted to marginal soils demonstrate exceptional tolerance to manganese limitation. Ann. Bot. 2019, 123, 831–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, A.; Iino, T.; Sonoike, K.; Miwa, E.; Higuchi, K. Remodeling of the major light-harvesting antenna protein of PSII protects the young leaves of barley (Hordeum vulgare L.) from photoinhibition under prolonged iron deficiency. Plant Cell Physiol. 2010, 51, 2013–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage-T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higuchi, K.; Kurita, K.; Sakai, T.; Suzui, N.; Sasaki, M.; Katori, M.; Wakabayashi, Y.; Majima, Y.; Saito, A.; Ohyama, T.; et al. “Live-Autoradiography” Technique Reveals Genetic Variation in the Rate of Fe Uptake by Barley Cultivars. Plants 2022, 11, 817. https://doi.org/10.3390/plants11060817
Higuchi K, Kurita K, Sakai T, Suzui N, Sasaki M, Katori M, Wakabayashi Y, Majima Y, Saito A, Ohyama T, et al. “Live-Autoradiography” Technique Reveals Genetic Variation in the Rate of Fe Uptake by Barley Cultivars. Plants. 2022; 11(6):817. https://doi.org/10.3390/plants11060817
Chicago/Turabian StyleHiguchi, Kyoko, Keisuke Kurita, Takuro Sakai, Nobuo Suzui, Minori Sasaki, Maya Katori, Yuna Wakabayashi, Yuta Majima, Akihiro Saito, Takuji Ohyama, and et al. 2022. "“Live-Autoradiography” Technique Reveals Genetic Variation in the Rate of Fe Uptake by Barley Cultivars" Plants 11, no. 6: 817. https://doi.org/10.3390/plants11060817