Mobile Host mRNAs Are Translated to Protein in the Associated Parasitic Plant Cuscuta campestris
Abstract
:1. Introduction
2. Results
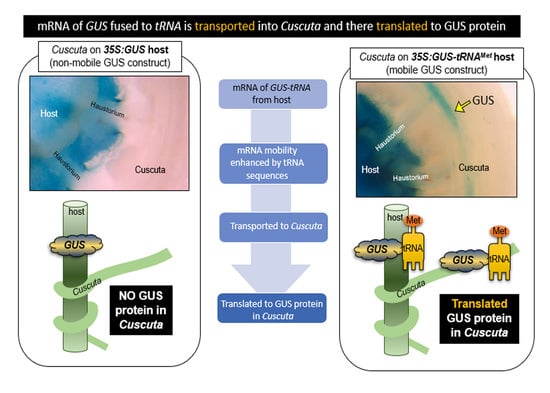
2.1. tRNA Fusions Influence Mobility of GUS Activity
2.2. GUS mRNA in C. campestris Is Associated with GUS-tRNA Fusions
2.3. GUS mRNA Moves Long Distances in C. campestris and GUS Activity Is Localized in Phloem Cells
3. Discussion
4. Material and Methods
4.1. Plant Material and Growth Conditions
4.2. Arabidopsis Plants Expressing ER-GUS with tRNAs
4.3. Histochemical and Quantitative GUS Assays
4.4. Paraffin Embedding
4.5. Phloroglucinol-HCl (Wiesner) Staining
4.6. Reverse Transcriptase (RT) PCR and Quantitative PCR
4.7. Aniline-Blue Staining
4.8. Searching tRNA-like Structure (TLS) Motif in Mobile Host Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Costea, M.; Tardif, F.J. The biology of Canadian weeds. 133. Cuscuta campestris Yuncker, C. gronovii Willd. ex Schult., C. umbrosa Beyr. ex Hook., C. epithymum (L.) L. and C. epilinum Weihe. Can. J. Plant Sci. 2006, 86, 293–316. [Google Scholar] [CrossRef]
- Shimizu, K.; Aoki, K. Development of parasitic organs of a stem holoparasitic plant in genus Cuscuta. Front. Plant Sci. 2019, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Shen, G.; Xu, Y.; Liu, H.; Zhang, J.; Li, S.; Li, J.; Zhang, C.; Qi, J.; Wang, L.; et al. Extensive inter-plant protein transfer between Cuscuta parasites and their host plants. Mol. Plant 2019, 13, 573–585. [Google Scholar] [CrossRef]
- Kim, G.; Westwood, J.H. Macromolecule exchange in Cuscuta–host plant interactions. Curr. Opin. Plant Biol. 2015, 26, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, N.R.; Axtell, M.J. Small RNA warfare: Exploring origins and function of trans-species microRNAs from the parasitic plant Cuscuta. Curr. Opin. Plant Biol. 2019, 50, 76–81. [Google Scholar] [CrossRef]
- Yang, Z.; Wafula, E.K.; Kim, G.; Shahid, S.; McNeal, J.R.; Ralph, P.E.; Timilsena, P.R.; Yu, W.-B.; Kelly, E.A.; Zhang, H.; et al. Convergent horizontal gene transfer and cross-talk of mobile nucleic acids in parasitic plants. Nat. Plants 2019, 2019. 5, 991–1001. [Google Scholar] [CrossRef]
- Kehr, J.; Buhtz, A. Long distance transport and movement of RNA through the phloem. J. Exp. Bot. 2007, 59, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.-J.; Huang, N.-C.; Liu, Y.-S.; Lu, C.-A.; Yu, T.-S. Long-distance movement of Arabidopsis FLOWERING LOCUS T RNA participates in systemic floral regulation. RNA Biol. 2012, 9, 653–662. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Canio, W.; Kessler, S.; Sinha, N. Developmental Changes Due to Long-Distance Movement of a Homeobox Fusion Transcript in Tomato. Science 2001, 293, 287–289. [Google Scholar] [CrossRef]
- Banerjee, A.K.; Chatterjee, M.; Yu, Y.; Suh, S.-G.; Miller, W.A.; Hannapel, D.J. Dynamics of a mobile RNA of potato involved in a long-distance signaling pathway. Plant Cell 2006, 18, 3443–3457. [Google Scholar] [CrossRef] [Green Version]
- Haywood, V.; Yu, T.-S.; Huang, N.-C.; Lucas, W.J. Phloem long-distance trafficking of GIBBERELLIC ACID-INSENSITIVE RNA regulates leaf development. Plant J. 2005, 42, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Hannapel, D.J. Long-Distance Signaling via Mobile RNAs. In Long-Distance Systemic Signaling and Communication in Plants; Baluška, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 53–70. [Google Scholar]
- Kehr, J.; Kragler, F. Long distance RNA movement. New Phytol. 2018, 218, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Thieme, C.J.; Rojas-Triana, M.; Stecyk, E.; Schudoma, C.; Zhang, W.; Yang, L.; Miñambres, M.; Walther, D.; Schulze, W.X.; Paz-Ares, J.; et al. Endogenous Arabidopsis messenger RNAs transported to distant tissues. Nat. Plants 2015, 1, 15025. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Mao, L.Y.; Jittayasothorn, Y.; Kang, Y.M.; Jiao, C.; Fei, Z.J.; Zhong, G.-Y. Messenger RNA exchange between scions and rootstocks in grafted grapevines. BMC Plant Biol. 2015, 15, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.H.; Liu, W.Q.; Wang, T.; Zhang, J.L.; Li, X.J.; Zhang, W.N. Systemic long-distance signaling and communication between rootstock and scion in grafted vegetables. Front. Plant Sci. 2020, 11, 460. [Google Scholar] [CrossRef]
- Kim, G.; LeBlanc, M.L.; Wafula, E.K.; de Pamphilis, C.W.; Westwood, J.H. Genomic-scale exchange of mRNA between a parasitic plant and its hosts. Science 2014, 345, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.K.; Lin, T.; Hannapel, D.J. Untranslated regions of a mobile transcript mediate RNA metabolism. Plant Physiol. 2009, 151, 1831–1843. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Perrera, V.; Saplaoura, E.; Apelt, F.; Bahin, M.; Kramdi, A.; Olas, J.; Mueller-Roeber, B.; Sokolowska, E.; Zhang, W.; et al. m5C methylation guides systemic transport of messenger RNA over graft junctions in plants. Curr. Biol. 2019, 29, 2465–2476.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Thieme, C.J.; Kollwig, G.; Apelt, F.; Yang, L.; Winter, N.; Andresen, N.; Walther, D.; Kragler, F. tRNA-Related Sequences Trigger Systemic mRNA Transport in Plants. Plant Cell 2016, 28, 1237–1249. [Google Scholar] [CrossRef] [Green Version]
- Schoenbeck, M.A.; Swanson, G.A.; Brommer, S.J. β-Glucuronidase activity in seedlings of the parasitic angiosperm Cusctua pentagona: Developmental impact of the β-glucuronidase inhibitor saccharic acid 1,4-lactone. Funct. Plant Biol. 2007, 34, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Waigmann, E.; Zambryski, P. Tobacco mosaic virus movement protein-mediated protein transport between trichome cells. Plant Cell 1995, 7, 2069–2079. [Google Scholar]
- Crawford, K.M.; Zambryski, P.C. Subcellular localization determines the availability of non-targeted proteins to plasmodesmatal transport. Curr. Biol. 2000, 10, 1032–1040. [Google Scholar] [CrossRef] [Green Version]
- Stadler, R.; Wright, K.M.; Lauterbach, C.; Amon, G.; Gahrtz, M.; Feuerstein, A.; Oparka, K.J.; Sauer, N. Expression of GFP-fusions in Arabidopsis companion cells reveals non-specific protein trafficking into sieve elements and identifies a novel post-phloem domain in roots. Plant J. 2005, 41, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Liljegren, S. Phloroglucinol stain for lignin. Cold Spring Harb. Protoc. 2010, 2010, pdb.prot4954. [Google Scholar] [CrossRef]
- Ruiz-Medrano, R.; Xoconostle-Cazares, B.; Lucas, W. Phloem long-distance transport of CmNACP mRNA: Implications for supracellular regulation in plants. Development 1999, 126, 4405–4419. [Google Scholar] [CrossRef]
- Lucas, W.J.; Yoo, B.-C.; Kragler, F. RNA as a long-distance information macromolecule in plants. Nat. Rev. Mol. Cell Biol. 2001, 2, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, L.; Kragler, F. The phloem-delivered RNA pool contains small noncoding RNAs and interferes withtranslation. Plant Physiol. 2009, 150, 378–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderwood, A.; Kopriva, S.; Morris, R.J. Transcript abundance axplains mRNA mobility data in Arabidopsis thaliana. Plant Cell 2016, 28, 610–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haupt, S.; Oparka, K.J.; Sauer, N.; Neumann, S. Macromolecular trafficking between Nicotiana tabacum and the holoparasite Cuscuta reflexa. J. Exp. Bot. 2001, 52, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Birschwilks, M.; Haupt, S.; Hofius, D.; Neumann, S. Transfer of phloem-mobile substances from the host plants to the holoparasite Cuscuta sp. J. Exp. Bot. 2006, 57, 911–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Qu, F.; Li, Z.; Doohan, D. Inter-species protein trafficking endows dodder (Cuscuta pentagona) with a host-specific herbicide-tolerant trait. New Phytol. 2013, 198, 1017–1022. [Google Scholar] [CrossRef]
- Chen, Q.; Payyavula, R.S.; Chen, L.; Zhang, J.; Zhang, C.; Turgeon, R. FLOWERING LOCUS T mRNA is synthesized in specialized companion cells in Arabidopsis and Maryland Mammoth tobacco leaf veins. Proc. Natl. Acad. Sci. USA 2018, 115, 2830–2835. [Google Scholar] [CrossRef] [Green Version]
- Slewinski, T.L.; Zhang, C.; Turgeon, R. Structural and functional heterogeneity in phloem loading and transport. Front. Plant Sci. 2013, 4, 244. [Google Scholar] [CrossRef] [Green Version]
- Shahid, S.; Kim, G.; Johnson, N.R.; Wafula, E.; Wang, F.; Coruh, C.; Bernal-Galeano, V.; Phifer, T.; de Pamphilis, C.W.; Westwood, J.H.; et al. MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature 2018, 553, 82–85. [Google Scholar] [CrossRef]
- Hozumi, A.; Bera, S.; Fujiwara, D.; Obayashi, T.; Yokoyama, R.; Nishitani, K.; Aoki, K. Arabinogalactan proteins accumulate in the cell walls of searching hyphae of the stem parasitic plants, Cuscuta campestris and Cuscuta japonica. Plant Cell Physiol. 2017, 58, 1868–1877. [Google Scholar] [CrossRef]
- Lao, J.; Oikawa, A.; Bromley, J.R.; McInerney, P.; Suttangkakul, A.; Smith-Moritz, A.M.; Plahar, H.; Chiu, T.-Y.; Fernández-Niño, S.M.G.; Ebert, B.; et al. The plant glycosyltransferase clone collection for functional genomics. Plant J. 2014, 79, 517–529. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Mitra, P.P.; Loqué, D. Histochemical Staining of Arabidopsis thaliana Secondary Cell Wall Elements. J. Vis. Exp. JoVE 2014, 87, 51381. [Google Scholar] [CrossRef] [Green Version]
- Guan, D.; Yan, B.; Thieme, C.; Hua, J.; Zhu, H.; Boheler, K.R.; Zhao, Z.; Kragler, F.; Xia, Y.; Zhang, S. PlaMoM: A comprehensive database compiles plant mobile macromolecules. Nucleic. Acids Res. 2017, 45, D1021–D1028. [Google Scholar] [CrossRef]




| Arabidopsis Lines | Number of Cuscuta Haustoria | Total Number of Samples | % with GUS Detection | |
|---|---|---|---|---|
| GUS Detected | No GUS | |||
| Wild type Col-0 | 0 | 13 | 13 | 0 |
| 35S:empty (pEarleygate100) | 0 | 10 | 10 | 0 |
| 35S:GUS | 0 | 35 | 35 | 0 |
| 35S:GUS-tRNAMET | 39 | 10 | 49 | 80 |
| 35S:GUS-tRNAMET dDT | 14 | 20 | 34 | 41 |
| 35S:GUS-tRNAGly | 12 | 20 | 32 | 38 |
| 35S:GUS-tRNAIle | 11 | 26 | 37 | 30 |
| 35S:GUS-tRNAMET dAT | 0 | 12 | 12 | 0 |
| 35S:ER-GUS | 0 | 12 | 12 | 0 |
| 35S:ER-GUS-tRNAMET | 9 | 17 | 26 | 35 |
| 35S:ER-GUS-tRNAMET dDT | 13 | 20 | 33 | 39 |
| 35S:ER-GUS-tRNAGly | 11 | 17 | 28 | 39 |
| 35S:ER-GUS-tRNAIle | 8 | 19 | 27 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Shimizu, K.; Brown, J.; Aoki, K.; Westwood, J.H. Mobile Host mRNAs Are Translated to Protein in the Associated Parasitic Plant Cuscuta campestris. Plants 2022, 11, 93. https://doi.org/10.3390/plants11010093
Park S-Y, Shimizu K, Brown J, Aoki K, Westwood JH. Mobile Host mRNAs Are Translated to Protein in the Associated Parasitic Plant Cuscuta campestris. Plants. 2022; 11(1):93. https://doi.org/10.3390/plants11010093
Chicago/Turabian StylePark, So-Yon, Kohki Shimizu, Jocelyn Brown, Koh Aoki, and James H. Westwood. 2022. "Mobile Host mRNAs Are Translated to Protein in the Associated Parasitic Plant Cuscuta campestris" Plants 11, no. 1: 93. https://doi.org/10.3390/plants11010093