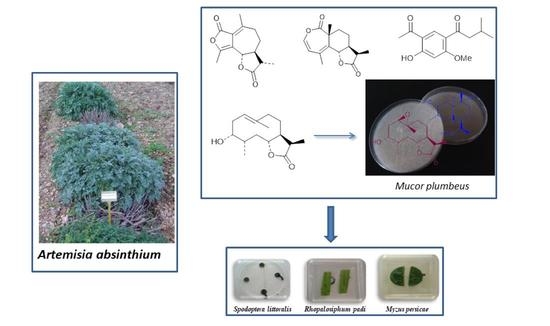
Sesquiterpene Lactones from Artemisia absinthium. Biotransformation and Rearrangement of the Insect Antifeedant 3α-hydroxypelenolide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Components of A. absinthium


2.2. Biotransformation and Rearrangement of 3α-hydroxypelenolide (3)
2.3. Biological Activity of 3α-hydroxypelenolide and Biotransformed Products 9 and 10
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Biodirected Chemical Fractionation
3.3.2. Absilactone (1)
3.3.3. Hansonlactone (2)
3.3.4. 3α-hydroxypelenolide (3)
3.3.5. Espeletone (11)
3.3.6. Ajenjol (12)
3.3.7. A Benzofuran Derivative (19)
3.4. Biotransformation and Rearrangement
3.4.1. Microorganism
3.4.2. Incubation of 3α-hydroxypelenolide (3)
3.4.3. 1β,10α-Epoxy-3α-hydroxypelenolide (9)
3.4.4. 1(R), 4(S), 6(S), 7(R), 11(R)-3α, 10α-Dihydroxy-cadinan-12-oic acid (10)
3.5. Insect Bioassays
3.5.1. Choice Feeding Assays
3.5.2. Oral Cannulation
3.6. Cytotoxicity
3.7. Phytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ariño, A.; Arberas, I.; Renobales, G.; Arriaga, S.; Dominguez, J.B. Essential Oil ofArtemisia absinthiumL. from the Spanish Pyrenees. J. Essent. Oil Res. 1999, 11, 182–184. [Google Scholar] [CrossRef]
- Julio, L.F.; Burillo, J.; Giménez, C.; Cabrera, R.; Díaz, C.E.; Sanz, J.; González-Coloma, A. Chemical and biocidal characterization of two cultivated Artemisia absinthium populations with different domestication levels. Ind. Crops Prod. 2015, 76, 787–792. [Google Scholar] [CrossRef]
- Julio, L.F.; Burgueño-Tapia, E.; Díaz, C.E.; Pérez-Hernández, N.; González-Coloma, A.; Joseph-Nathan, P. Absolute configuration of the ocimene monoterpenoids fromArtemisia absinthium. Chirality 2017, 29, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Suchý, M.; Samek, Z.; Herout, V.; Bates, R.B.; Snatzke, G.; Šorm, F. On terpenes. CLXXXVIII. Constitution and configuration of pelenolides, a new group of sesquiterpene lactone germacranolides. Collect. Czechoslov. Chem. Commun. 1967, 32, 3917–3925. [Google Scholar] [CrossRef]
- Bates, R.B.; Cheer, C.J.; Sneath, T.C. Crystal structure of hydroxypelenolide p-bromobenzoate. J. Org. Chem. 1970, 35, 3960–3961. [Google Scholar] [CrossRef]
- Gonzalez-Coloma, A.; Bailen, M.; Diaz, C.E.; Fraga, B.M.; Martínez-Díaz, R.; Zuñiga, G.E.; Contreras, R.A.; Cabrera, R.; Burillo, J. Major components of Spanish cultivated Artemisia absinthium populations: Antifeedant, antiparasitic, and antioxidant effects. Ind. Crops Prod. 2012, 37, 401–407. [Google Scholar] [CrossRef]
- Aberham, A.; Cicek, S.S.; Schneider, P.; Stuppner, H. Analysis of Sesquiterpene Lactones, Lignans, and Flavonoids in Wormwood (Artemisia absinthiumL.) Using High-Performance Liquid Chromatography (HPLC)−Mass Spectrometry, Reversed Phase HPLC, and HPLC−Solid Phase Extraction−Nuclear Magnetic Resonance. J. Agric. Food Chem. 2010, 58, 10817–10823. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Toda, Y.; Kato, K.; Miyamoto, K.; Hasegawa, T.; Yamada, K.; Ueda, J.; Hasegawa, K.; Inoue, T.; Shigemori, H. Artabolide, a novel polar auxin transport inhibitor isolated from Artemisia absinthium. Tetrahedron 2013, 69, 7001–7005. [Google Scholar] [CrossRef] [Green Version]
- Bhatti, H.N.; Khera, R.A. Biotransformations of diterpenoids and triterpenoids: A review. J. Asian Nat. Prod. Res. 2013, 16, 70–104. [Google Scholar] [CrossRef]
- Bailen, M.; Martínez-Díaz, R.A.; Hoffmann, J.J.; Gonzalez-Coloma, A. Molecular Diversity from Arid-Land Plants: Valorization of Terpenes and Biotransformation Products. Chem. Biodivers. 2020, 17, 1900663. [Google Scholar] [CrossRef]
- Arantes, S.; Hanson, J. The Biotransformation of Sesquiterpenoids by Mucor plumbeus. Curr. Org. Chem. 2007, 11, 657–663. [Google Scholar] [CrossRef]
- Silva, E.D.O.; Furtado, N.A.J.C.; Aleu, J.; Collado, I.G. Terpenoid biotransformations by Mucor species. Phytochem. Rev. 2013, 12, 857–876. [Google Scholar] [CrossRef]
- Fraga, B.M.; Gonzalez, P.; Guillermo, R.; Hernandez, M.G. Microbiological Transformation of Manoyl Oxide Derivatives byMucorplumbeus. J. Nat. Prod. 1998, 61, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Fraga, B.M.; Hernández, M.G.; Gonzalez, P.; López, M.; Suarez, S. Biotransformation of the diterpene ribenone by Mucor plumbeus. Tetrahedron 2001, 57, 761–770. [Google Scholar] [CrossRef]
- Fraga, B.M.; Hernández, M.G.; Artega, J.M.; Suárez, S. The microbiological transformation of the diterpenes dehydroabietanol and teideadiol by Mucor plumbeus. Phytochemistry 2003, 63, 663–668. [Google Scholar] [CrossRef]
- Fraga, B.M.; Guillermo, R.; Hernandez, M.G.; Chamy, M.C.; Garbarino, J.A.; Chamy, M.C. Biotransformation of two stemodane diterpenes by Mucor plumbeus. Tetrahedron 2004, 60, 7921–7932. [Google Scholar] [CrossRef]
- Fraga, B.M.; De Alfonso, I.; Gonzalez-Vallejo, V.; Guillermo, R. Microbial transformation of two 15α-hydroxy-ent-kaur-16-ene diterpenes by Mucor plumbeus. Tetrahedron 2010, 66, 227–234. [Google Scholar] [CrossRef]
- Fraga, B.M.; Díaz, C.E.; Amador, L.J.; Reina, M.; López-Rodriguez, M.; González-Coloma, A. Biotransformation of an africanane sesquiterpene by the fungus Mucor plumbeus. Phytochemistry 2017, 135, 73–79. [Google Scholar] [CrossRef]
- Chi, J.; Li, B.-C.; Dai, W.-F.; Liu, L.; Zhang, M. Highly oxidized sesquiterpenes from Artemisia austro-yunnanensis. Fitoterapy 2016, 115, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, F.; Jakupovic, J.; Dutta, L.; Goodman, M. Neue, abgewandelte pseudoguajanolide aus Psilostrophe villosa. Phytochemistry 1980, 19, 1491–1494. [Google Scholar] [CrossRef]
- Bohlmann, F.; Rao, N. Neue Hydroxyacetophenon-Derivate ausEspeletia schultzii Wedd. Eur. J. Inorg. Chem. 1973, 106, 3035–3038. [Google Scholar] [CrossRef]
- Takasugi, M.; Masuda, T. Three 4′-hydroxyacetophenone-related phytoalexins from Polymnia sonchifolia. Phytochemistry 1996, 43, 1019–1021. [Google Scholar] [CrossRef]
- The Components of Cacalia tangutica. Bull. Korean Chem. Soc. 2004, 25, 1078–1080. [CrossRef] [Green Version]
- Castañeda, P.; Gómez, L.; Mata, R.; Lotina-Hennsen, B.; Anaya, A.L.; Bye, R. Phytogrowth-Inhibitory and Antifungal Constituents ofHelianthella quinquenervis. J. Nat. Prod. 1996, 59, 323–326. [Google Scholar] [CrossRef]
- González, A.; Bermejo, J.; Estévez, F.; Velázquez, R. Phenolic derivatives from Artemisia glutinosa. Phytochemistry 1983, 22, 1515–1516. [Google Scholar] [CrossRef]
- Tsankova, E.; Bohlmann, F. A monoterpene from Aster bakeranus. Phytochemistry 1983, 22, 1285–1286. [Google Scholar] [CrossRef]
- Wang, W.; Tan, R.; Yao, Y.; Wang, Q.; Jiang, F. Sesquiterpene lactones from Ajania fruticulosa. Phytochemistry 1994, 37, 1347–1349. [Google Scholar] [CrossRef]
- Chamy, M.C.; Piovano, M.; Gambaro, V.; Garbarino, J.A.; Nicoletti, M. Dehydroabietane diterpenoids from Calceolaria ascendens. Phytochemistry 1987, 26, 1763–1765. [Google Scholar] [CrossRef]
- Öksüz, S.; Ulubelen, A.; Barla, A.; Voelter, W. Terpenoids and aromatic compounds from Euphorbia heteradena. Turk. J. Chem. 2002, 26, 457–463. [Google Scholar]
- Talapatra, B.; Chaudhuri, P.K.; Mallik, A.K.; Talapatra, S.K. Lagerenyl acetate and lagerenol two tetracyclic triterpenoids with the cycloartane skeleton from Lagerstroemia lancasteri. Phytochemistry 1983, 22, 2559–2562. [Google Scholar] [CrossRef]
- Della Greca, M.; Fiorention, A.; Monaco, P.; Previtera, L. Cycloartane triterpenes from Juncus effusus. Phytochemistry 1994, 35, 1017–1022. [Google Scholar] [CrossRef]
- Bohlmann, F.; Le Van, N. Neue Guajanolide aus Podachaenium eminens. Phytochemistry 1977, 16, 1304–1306. [Google Scholar] [CrossRef]
- Ahmed, D.; Choudhary, M.; Turkoz, S.; Şener, B.; Rahman, A.-U. Chemical Constituents of Buxus sempervirens. Planta Med. 1988, 54, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Inokuchi, T.; Fujioka, S.; Kimura, Y. Phenolic Compounds and Flavonoids as Plant Growth Regulators from Fruit and Leaf of Vitex rotundifolia. Z. Nat. C 2004, 59, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asker, E.; Akin, S.; Hökelek, T. 5,3′-Dihydroxy-3,6,7,4′-tetramethoxyflavone. ACTA Crystallogr. Sect. E Struct. Rep. Online 2006, 62. [Google Scholar] [CrossRef]
- Milosavjević, S.; Aljančić, I.; Macura, S.; Milinkovic, D.; Stefanović, M. Sesquiterpene lactones from Achillea crithmifolia. Phytochemistry 1991, 30, 3464–3466. [Google Scholar] [CrossRef]
- Milosavljevic, S.; Juranic, I.; Aljancic, I.; Vajs, V.; Todorovic, N. Conformation analysis of three germacranolides by the PM3 semi-empirical method. J. Serb. Chem. Soc. 2003, 68, 281–289. [Google Scholar] [CrossRef]
- Thormann, U.; De Mieri, M.; Neuburger, M.; Verjee, S.; Altmann, P.; Hamburger, M.; Imanidis, G. Mechanism of Chemical Degradation and Determination of Solubility by Kinetic Modeling of the Highly Unstable Sesquiterpene Lactone Nobilin in Different Media. J. Pharm. Sci. 2014, 103, 3139–3152. [Google Scholar] [CrossRef] [PubMed]
- Depascualt, J.; Gonzalez, M.; Valle, M.; Bellido, I. Heliangolides from Leucanthemopsis pulverulenta. Phytochemistry 1983, 22, 1985–1987. [Google Scholar] [CrossRef]
- Teresa, J.D.P.; Valle, M.M.; Gonzalez, M.; Bellido, I. Sesquiterpenic acids from Leucanthemopsis pulverulenta. Tetrahedron 1984, 40, 2189–2195. [Google Scholar] [CrossRef]
- Teresa, J.D.P.; Gonzalez, M.; Caballero, M.; Parra, T.; Bellido, I. Transannular cyclization of heliangolides. Tetrahedron Lett. 1987, 28, 821–824. [Google Scholar] [CrossRef]
- Moujir, L.; Callies, O.; Sousa, P.M.C.; Sharopov, F.; Seca, A.M.L. Applications of Sesquiterpene Lactones: A Review of Some Potential Success Cases. Appl. Sci. 2020, 10, 3001. [Google Scholar] [CrossRef]
- Wu, H.-B.; Wang, W.-S.; Liu, T.-T.; Qi, M.-G.; Feng, J.-C.; Li, X.-Y.; Liu, Y.; Wu, H.-B. Insecticidal activity of sesquiterpene lactones and monoterpenoid from the fruits of Carpesium abrotanoides. Ind. Crops Prod. 2016, 92, 77–83. [Google Scholar] [CrossRef]
- Macias, F.A.; Galindo, J.C.G.; Castellano, D.; Velasco, R.F. Sesquiterpene Lactones with Potential Use as Natural Herbicide Models (I): Trans,trans-Germacranolides. J. Agric. Food Chem. 1999, 47, 4407–4414. [Google Scholar] [CrossRef]
- Buchanan, O.G.; Williams, L.A.; Reese, P.B. Biotransformation of cadinane sesquiterpenes by Beauveria bassiana ATCC 7159. Phytochemistry 2000, 54, 39–45. [Google Scholar] [CrossRef]
- Ro, D.-K.; Paradise, E.M.; Ouellet, M.; Fisher, K.J.; Newman, K.L.; Ndungu, J.M.; Ho, K.A.; Eachus, R.A.; Ham, T.S.; Kirby, J.; et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nat. Cell Biol. 2006, 440, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Nawamaki, K.; Kuroyanagi, M. Sesquiterpenoids from Acorus calamus as germination inhibitors. Phytochemistry 1996, 43, 1175–1182. [Google Scholar] [CrossRef]
- Julio, L.F.; Barrero, A.F.; Del Pino, M.M.H.; Arteaga, J.F.; Burillo, J.; Andres, M.F.; Díaz, C.E.; González-Coloma, A. Phytotoxic and Nematicidal Components of Lavandula luisieri. J. Nat. Prod. 2016, 79, 261–266. [Google Scholar] [CrossRef]
- Burillo, J. Cultivo experimental de ajenjo Artemisia absinthium L. como potencial insecticida de origen natural. In Insecticidas y Repelentes de Origen Natural; Centro de Investigación y Tecnología Agroalimentaria: Zaragoza, Spain, 2009; pp. 19–30. [Google Scholar]
- Coll, J.C.; Bowden, B.F. The Application of Vacuum Liquid Chromatography to the Separation of Terpene Mixtures. J. Nat. Prod. 1986, 49, 934–936. [Google Scholar] [CrossRef]
- Reina, M.; González-Coloma, A.; Gutiérrez, C.; Cabrera, R.; Rodríguez, M.L.; Fajardo, V.; Villarroel, L. Defensive Chemistry ofSenecio miser. J. Nat. Prod. 2000, 64, 6–11. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- González-Coloma, A.; Guadaño, A.; De Inés, C.; Martínez-Díaz, R.; Cortes, D. Selective Action of Acetogenin Mitochondrial Complex I Inhibitors. Z. Nat. C 2002, 57, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Moiteiro, C.; Curto, M.J.M.; Mohamed, N.; Bailén, M.; Martínez-Díaz, R.; González-Coloma, A. Biovalorization of Friedelane Triterpenes Derived from Cork Processing Industry Byproducts. J. Agric. Food Chem. 2006, 54, 3566–3571. [Google Scholar] [CrossRef] [PubMed]

| Carbon | 1 | 2 | 3 | 9 | 10 a | 11 | 12 | 14 | 19 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 121.2 | 168.8 | 123.3 | 59.0 | 45.8 | 128.9 | 113.8 | 128.5 | - |
| 2 | 166.8 | 138.8 | 34.4 | 34.1 | 34.4 | 133.2 | 167.5 | 136.1 | 164.8 |
| 3 | - | 117.3 | 73.6 | 71.3 | 71.2 | 111.5 | 99.7 | 118.7 | 100.9 |
| 4 | 149.0 | 127.9 | 40.2 | 38.0 | 37.5 | 161.6 | 164.8 | 166.3 | 122.3 |
| 5 | 111.6 | 129.6 | 38.6 | 36.0 | 33.9 | 130.2 | 120.9 | 119.2 | 132.8 |
| 6 | 78.3 | 80.1 | 86.1 | 85.3 | 42.1 | 131.1 | 135.1 | 131.2 | 124.9 |
| 7 | 49.0 | 45.3 | 47.3 | 47.0 | 47.9 | 196.4 | 203.4 | 195.7 | 111.2 |
| 8 | 26.0 | 23.2 | 27.3 | 24.0 | 26.2 | 26.4 | 26.2 | 26.3 | 157.4 |
| 9 | 37.6 | 32.0 | 40.4 | 39.3 | 43.4 | 202.0 | 199.4 | 206.8 | 128.5 |
| 10 | 154.4 | 48.0 | 135.8 | 60.5 | 72.8 | 52.6 | 52.6 | 47.0 | 69.4 |
| 11 | 41.5 | 42.5 | 37.0 | 37.0 | 40.6 | 24.9 | 24.9 | 25.3 | 28.8 |
| 12 | 177.6 | 178.4 | 179.4 | 180.2 | 179.5 | 22.7 | 22.7 | 22.7 | 28.8 |
| 13 | 12.4 | 12.6 | 10.7 | 10.4 | 15.2 | 22.7 | 22.7 | 22.7 | 197.6 |
| 14 | 22.6 | 19.8 | 16.3 | 17.0 | 21.0 | 55.9 | 55.9 | 26.7 | |
| 15 | 13.5 | 21.3 | 19.3 | 22.1 | 19.2 | - | - |
| Compound | S. littoralis | pANCOVA2 (ΔI covariate) | Sf9 EC50 [μg/mL] | |
|---|---|---|---|---|
| ΔB a | ΔI b | |||
| 3 | 97 | 94 | 29.5 (19.2, 45.5) | |
| 9 | 87 * | 92 | >100 | |
| 10 | 79 * | 81 * | p = 0.51 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraga, B.M.; Díaz, C.E.; Bailén, M.; González-Coloma, A. Sesquiterpene Lactones from Artemisia absinthium. Biotransformation and Rearrangement of the Insect Antifeedant 3α-hydroxypelenolide. Plants 2021, 10, 891. https://doi.org/10.3390/plants10050891
Fraga BM, Díaz CE, Bailén M, González-Coloma A. Sesquiterpene Lactones from Artemisia absinthium. Biotransformation and Rearrangement of the Insect Antifeedant 3α-hydroxypelenolide. Plants. 2021; 10(5):891. https://doi.org/10.3390/plants10050891
Chicago/Turabian StyleFraga, Braulio M., Carmen E. Díaz, María Bailén, and Azucena González-Coloma. 2021. "Sesquiterpene Lactones from Artemisia absinthium. Biotransformation and Rearrangement of the Insect Antifeedant 3α-hydroxypelenolide" Plants 10, no. 5: 891. https://doi.org/10.3390/plants10050891