1. Introduction
Within their lifetime, about 15% of patients with diabetes mellitus are bound to develop foot ulcerations, a chronic condition of delayed healing which often leads to amputation, thereby causing the patient to be burdened with significant morbidity and mortality. Indeed, diabetes is the leading cause of non-traumatic limb amputation in western countries, and the mortality after a major amputation is similar to or worse than that of several malignant cancers [
1]. Wound healing is a complex process involving several tissues and cell types, and is characterized by different and coordinated phases, including inflammation, coagulation, fibrogenesis, the formation of the granulation tissue through angiogenesis, fibrinogenesis, and its regression [
2]. Any alterations to the above-described events can delay healing and lead to wound complications.
The mechanisms responsible for the delayed healing of ulcers in diabetes are manifold and not yet fully understood [
3]. In a recent proteomic study carried out on ulcer tissue samples from patients with diabetes, the serpin B3 (SB3) protein was identified as a positive biomarker of successful healing. Serpin B3 is a protease inhibitor involved in the activation of fibrogenesis and angiogenesis, which are two key processes of wound resolution [
4,
5]. SB3 was strongly downregulated in non-healing diabetic wounds as compared to rapidly healing wounds, and such a finding was validated using proteins and mRNA from individual samples [
6,
7]. These findings prompted us to investigate whether the local administration of exogenous SB3 can improve diabetic wound healing. However, the topical administration of SB3 at an open ulcer presents some technical challenges due to the protein nature of this agent. In fact, the ulcer environment is normally aggressive to proteins (which are prone to inactivation secondary to proteolytic digestion and/or physical denaturation) and could quickly reduce local SB3 levels, unless a constant supply of functional protein is provided. In order to achieve this, a prolonged release of the bioactive agent to the site of action is desirable. In addition, the viscoelastic properties of the formulation should permit a long-time residence at the application point, without dripping or drying out. Finally, the vehicle should be capable of preserving the active agent’s stability over time to guarantee the shelf life.
Although the administration of proteins at the outer skin may be useful for some pathologies, to date, little effort has been devoted to the development of specific protein formulations that are suitable for this route [
8,
9,
10]. Indeed, most of the strategies for the delivery of proteins described in the literature are finalized to parenteral applications or local administration for the purpose of a transdermal or systemic effect [
11]. On the other hand, many of the solutions optimized for parenteral uses (liposomes, micelles, or inclusion in hydrogels) may be suitable for external application, provided that the release characteristics of the active element and the viscoelastic properties of the formulation administered are suitable for this use.
In particular, the use of hydrogel carriers may fit this purpose. Hydrogels (which can be obtained using a wide variety of polymeric materials) can both stabilize proteins and generate systems with adequate viscoelastic properties to permit physical retention at the site of administration [
12,
13,
14]. However, not many hydrogels are able to generate a slow and prolonged release of proteins, a property that is fundamental in order to provide a constant supply to the application site.
One possible solution may come from wet sol–gel silica-based vehicles [
15]. Sol–gel silica is a highly porous inorganic polymer obtained synthetically in mild conditions from silicon alkoxide precursors. Sol–gel silica polymerization occurs in aqueous/alcoholic environments, which, in some cases, are compatible with the stability of proteins [
16,
17,
18]. If the polymerization is carried out in the presence of a bioactive agent, the agent remains physically trapped within the newly generated polymer network.
The product of polymerization is a ‘wet’ monolith in which the silica network entrapping the bioactive agent is surrounded by an aqueous/alcoholic solution, the composition of which depends on the type and concentration of the reagents used. The initial gel can then be dried (xerogel or aerogel) or it can be used in the wet form [
18].
In the case of proteins, it has been shown that when they are embedded in wet sol–gel silica, they often maintain their folded state, they are protected from denaturation [
18,
19,
20,
21], and they can be slowly released (within days or weeks) secondary to diffusion and/or polymer erosion. Erosion, which occurs in aqueous environments, releases silicic acid—a component of mineral waters—which is biocompatible and nontoxic.
On the downside, sol–gel silica is not spreadable, and when exposed to air, it undergoes an irreversible syneresis, which reduces the polymer pore size and, thus, the speed of erosion and the diffusivity of the embedded proteins.
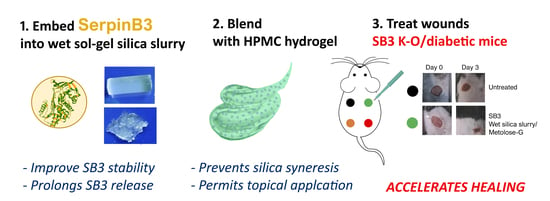
In order to overcome these limits, we investigated the possibility of blending a protein-loaded wet sol–gel silica with an organic hydrogel carrier, with the final goal of developing a formulation for the sustained delivery of SB3 to chronic ulcers.
Initially, we evaluated the effects of silica entrapment on SB3’s structure, biological activity, and release kinetics. A composite preparation suitable for topical application was then optimized by first crushing the wet sol–gel silica monolith into a wet gel slurry and then suspending it in a hydroxypropyl methyl cellulose (HPMC)-based hydrogel (
Figure 1). The effects of both crushing and HPMC blending on the protein-releasing properties of the silica gel were investigated. Following the pre-formulation studies, an optimized SB3-loaded formulation was then generated, and its potential for treating wounds was compared to that of SB3 in a buffer or in the HMPC hydrogel using two animal models characterized by impaired wound healing: SB3 knockout (KO) mice and streptozotocin (STZ)-induced diabetic mice.
2. Materials and Methods
2.1. Materials and Instrumentation
Human (h)SB3 was obtained in our laboratory using recombinant molecular techniques, as previously described [
22]. Mouse serpin B3 analogue (m)SB3 was purchased from NZYTech (Lisboa, Portugal); human anti-SB3 rabbit polyclonal Abs were kindly provided by Xeptagen S.p.A, (Vega Park, Venice, Italy); hydroxypropyl methyl cellulose (HPMC, code 90-SH-100000 SR) was obtained from Seppic (Milan, Italy); Kollidon 30 (# 63-0137) was obtained from BASF (Germany); pronase from
Streptomyces griseus (# 2555112) was obtained from Roche Diagnostics; ECL Prime Western blotting detection reagent (# 9995053) was obtained from GE Healthcare; and bovine serum albumin (BSA # 078K0710), tetramethyl orthosilicate (TMOS, # BCBN3603V), fluorescein isothiocyanate (FITC # 039K5310), electrophoresis marker C1992-1VL (# 108K6401, MW: 8000-222,000), and all other reagents were from Sigma Aldrich (Merck, St. Louis, MO, USA).
BSA–FITC was obtained through the conjugation of BSA with 10 equivalents of FITC in PBS. The product (FITC:BSA = 2:1 mol:mol) was purified by extensive dialysis (10 kDa cut-off) against PBS, sterile filtered, and maintained at 4 °C until use. Spectroscopic analyses were performed with a Varian Cary 50 UV-Vis spectrophotometer. The fluorescence was measured using a JASCO FP-6200 spectrofluorometer. Circular dichroism spectra were recorded using a JASCO J-810 spectropolarimeter. Protein release studies were conducted using 1 mL Float-A-Lyzer G2 dialysis tubes from Spectrum Laboratories, Inc. A Hettich Universal 320-R centrifuge was used for the gel washes. A Timo autoclave was used for sterilization. A Memmert GmbH, model SV 1422, was used as a thermostated bath. Electrophoresis on a polyacrylamide gel was conducted using a gel casting from the Bio-Rad Mini-Protean System. Western blots were performed on Millipore PVDF (polyvinylidene fluoride) membranes. The Molecular Imager VersaDoc MP4000 was used for the acquisitions of the Western blot membranes.
2.2. Preparation of Wet Sol–Gel Silica Gel Slurries
All steps were performed in a sterile environment to prevent microbial contamination. Gel monoliths (8% SiO
2 w/
v) were obtained from tetramethoxysilane (TMOS) according to a procedure already described [
18,
20]. Briefly, the partial hydrolysis of the alkoxysilane precursor (activated sol) was initially promoted with an acidic treatment at room temperature for 30 min using HCl (Si:HCl:H
2O molar ratios = 1:6 × 10
−6:1.25). For each mL of wet sol–gel silica, 230.4 µL of activated sol was mixed, under a gentle vortex, with 769.6 µL of protein solution in PBS + 0.1% Kollidon 30. When used as such, the monoliths were rinsed (3×) with 5 volumes of buffer (100 mM phosphate, pH 6.0) to extract the methanol liberated during condensation. The process allowed for the quantitative entrapment of the protein in solution. Wet gels were prepared at a protein load varying between 5 and 200 µg/mL of gel.
Wet gel slurries were obtained immediately after the monolith formation by manual crushing using a sterile glass rod, and were washed thoroughly (3×) by adding 5 volumes of buffer (100 mM phosphate, pH 6.0), centrifuging (1390× g, 1 min), and removing the supernatant.
The products were used immediately after preparation or stored at 4 °C and sealed with a minimum amount of buffer to prevent drying. For the in vivo administration, the gel slurry (200 µg of SB3/mL) was diluted to 1:200 into a sterile 3% HPMC hydrogel (metolose) containing 10% w/v glycerol (metolose-G).
2.3. Structural Conformation of SB3
The SB3’s protein conformation in solution and within the silica sol–gel was determined using circular dichroism. An analysis was carried out at 25 °C in a quartz cuvette with an optical path of 1 mm, and the following parameters were set: λ: 190–260 nm; bandwidth: 2 nm; response time: 16 s; sensitivity: standard; data pitch: 0.1 nm; scanning speed: 10 nm/min; and acquisitions: 2 (for blank) and 4 (for protein). The spectra in solution were registered at 0.15 mg/mL of SB3 in 100 mM phosphate with a pH of 6.0. The spectra in the wet gel (SB3 at 0.3 mg/mL of gel) were registered from the wet silica slurry suspended in an equal volume of buffer inside the quartz cuvette.
2.4. SB3 Thermal Stability
The protein’s t-melt in solution (0.15 mg/mL in 100 mM phosphate, pH 6.0) and when trapped within the wet gel matrix (0.3 mg/mL in the gel, diluted 1:1 with 100 mM phosphate, pH 6.0, for suspension) was determined by circular dichroism. Repeated CD spectra were acquired (between 200 and 260 nm with 0.1 nm intervals) at 10 °C intervals in a temperature range between 40 °C and 90 °C, with a heating rate of 60 °C/h. The thermal denaturation curves were obtained by plotting the mean ellipticity value per residue, measured at 222 nm as a function of the temperature of analysis.
2.5. SB3 Stability to Proteolysis
SB3 was analyzed by Western blot (after 12% SDS PAGE) after its release from a wet sol–gel silica slurry previously incubated with the proteolytic enzyme pronase. More into details, prior to release, the wet sol–gel silica gel slurry (200 µL) containing SB3 (300 µg/mL of gel) was suspended in 500 µL of pronase (3 µg/mL; SB3:pronase w/w ratio = 40:1) in HEPES (10 mM) + NaCl (150 mM) + CaCl2 (10 mM), with a pH of 7.4. After 1 h of incubation at 37 °C, the gel was washed 6 times with 100 mM phosphate with a pH of 6.0 to remove the pronase and was then incubated at 37 °C in 10 mL of release buffer (HEPES buffer (100 mM) + 0.1% BSA + 0.05% Tween 20, pH 7.4). After 1 h, the release solution was analyzed using SDS-PAGE and Western blot. The SB3 on the membrane was detected with ECL after incubation with rabbit anti-human SB3 antibodies, followed by goat anti-rabbit IgG HRP. Similarly, the protein in solution was incubated with pronase at an SB3:pronase w/w ratio = 40:1 for 1 h at 37 °C and then analyzed by WB. As a control, the same release protocol was carried out on an SB3–gel slurry that had not been treated with pronase.
2.6. Protein Release from Wet Silica Gel
The wet silica gel (200 µL of either monolith or slurry) was suspended at 37 °C in 10 mL of 100 mM phosphate buffer, 0.05% Tween 20, and 0.1% BSA at a pH of 6.0 and incubated at 37 °C under gentle shaking. At defined time points (0 h, 5 h, and 24 h), a small portion of the supernatant (250 µL) was removed for SB3 titration and replaced with fresh buffer. The gel was then re-suspended in the supernatant using a gentle vortex and the incubation was continued until the following time point. When testing the gel slurry, the sample was centrifuged (1390× g, 3 min) before supernatant isolation.
In order to evaluate the influence of HPMC hydrogel blending on the protein release from wet sol–gel silica, slurries embedded with BSA–FITC were suspended in an equal volume of release buffer 3% HPMC, inserted into dialysis tubes (cut-off of 100 kDa), and immersed in PBST + 0.1% BSA at 37 °C. At scheduled time points, the protein content in the acceptor solution was quantified.
BSA–FITC titration in the release medium was carried out using fluorescence spectroscopy. SB3 titration was performed with ELISA testing (HEPA Lisa kit, Xeptagen S.p.A, Vega Park, Venice, Italy) by following the manufacturer’s instructions. Briefly, the release solutions obtained from the gels loaded with SB3 at 5 or 50 µg/mL were diluted to 1:5 or 1:50, respectively, and were incubated for 1 h at room temperature on 96-well plates coated with rabbit anti-human SB3 capture Abs (10 μg/mL in PBS, pH 7.4). A standard curve, obtained through the dilution of recombinant SB3 from 16 to 0.25 ng/mL, was also included as a calibrator. After washing, SB3 was revealed through incubation with 100 μL of HRP-conjugated streptavidin secondary anti-SB3 Abs (0.5 μg/mL). The plate was developed with a ready-to-use 3,3′, 5,5′-tetramethylbenzidine (TMB) substrate solution. The reaction was stopped with 1 mol/L of HCl (100 μL) and the absorbance at 450 nm was measured on a microplate reader (Victor × 3; Perkin Elmer, Waltham, MA, USA). All samples were tested in duplicate. The results were graphically represented by plotting the ratio between the quantity of released protein at each time point (Mt) and the total protein (Mtot) embedded as a function of the time.
The activity of the released SB3 was verified with a scatter assay, as previously reported [
23]. In detail, Madin–Darby canine kidney cells (MDCK) (5 × 10
3 cells/well) were treated with two different dilutions of the release media (1:16 and 1:128) obtained after 0, 5, and 24 h of the release assay. Cells were fixed in 4% formaldehyde and stained with Coomassie blue solution, and the scattering effect was analyzed using a 20 Axiovert 200M microscope (Carl Zeiss Microscopy GmbH, Jena, Germany). As a control, positive recombinant SB3 was used at 200 ng/mL.
2.7. In Vivo Testing
Animal experiments were approved by the University of Animal Care Padua and the Italian Ministry of Health (Aut. No. 171/2019-PR). SB3 knockout mice [
24] were bred in-house. For the diabetic model, diabetes was induced using a single intraperitoneal injection of 150 mg/kg of streptozotocin (STZ) (Sigma Aldrich) in citrate buffer (50 mmol/l, pH 4.5). The blood glucose was measured with a Glucocard G-meter (Menarini, Florence, Italy); mice with a blood glucose level ≥ 16.7 mmol/L (300 mg/dL) in at least two measurements within the first week were classified as diabetic and housed for 4 weeks with free access to food and water before the experiments were performed. To create skin wounds, mice were sedated through sodium isoflurane inhalation, and the skin of the back was shaved with Veet cream and disinfected with povidone iodine at 10%. Then, 4 mm-diameter wounds were inflicted with a biopsy punch (H-S Medical, Colton, CA, USA).
The animal wounds were treated locally with 100 ng of SB3 formulated in 100 μL of 100 mM phosphate buffer + 0.1% Kollidon-30, with a pH of 6.0, or 100 μL of metolose-G or 100 μL of metolose-G containing wet silica slurry (metolose-G:silica slurry = 200:1 v:w) (SB3 in wet silica gel = 200 μg/mL). Metolose-G and an SB3-free wet silica slurry diluted by 200× in metolose were used as controls. Each treatment (N ≥ 7 for each group) was administered twice, immediately after the execution of the wound and the day after. During the observation period, no other wound dressing was applied. To evaluate the time to wound closure, photographs were taken at selected time points, and the wound area was quantified with ImageJ.
2.8. Statistical Analysis
Statistical analyses were carried out using the Graph Pad software (v. 8.0.2, San Diego, CA, USA). Two-way ANOVA was used to assess significance, which was considered for p-values below 0.05.
4. Discussion
An ideal vehicle for the topical delivery of proteins to open wounds should be characterized by a number of properties: (a) it should not be harmful to the structure (and biological activity) of the protein and, possibly, preserve its stability upon storage to favor long-lasting efficacy and a long shelf life; (b) it should allow for the slow release of the active agent to guarantee a constant supply at the site of administration; and (c) it should possess proper viscoelastic properties to permit a long-time residence at the application point, without dripping or drying out.
The wet sol–gel silica here investigated proved to have ideal features in terms of protein stabilization and SB3 release kinetics [
15,
19]. Indeed, the SB3 entrapment into the 8% SiO
2 silica gel did not alter its biological activity and allowed for its sustained release over at least 3 days, so that we could treat the animals with a single dose. Notably, since SB3 gel entrapment stabilized the protein against physical denaturation and proteolytic attack, the fraction of unreleased protein remained protected and active until released. Therefore, the silica gel acted as a stabilizing depot, providing a long-lasting supply of active protein along the entire release process.
An interesting result from the preliminary tests is that the shape and size of the silica gel did not affect the protein’s release kinetics. This may be explained, considering that the protein release from the wet sol–gel silica was mainly secondary to silica erosion, a phenomenon that is driven by water diffusing within the silica network. It is likely that, at the SiO2 concentration here used, the pores of the silica network were large enough not to impair the free diffusion of water molecules. As a consequence, silica erosion (and the protein release from it) occurred equally and at the same speed at both the periphery and from within the matrix. From a practical point of view, this result indicates that strict control over the size of the crushed particles is unnecessary.
The metolose-G blend used for suspending the sol–gel silica provided the textural properties necessary for topical application without affecting the protein release or stability. Both components were selected among others because of their extensive use in semisolid formulations for topical administration. Glycerol is a natural and highly biocompatible osmotic agent classically used as humectant and emollient in aqueous-based semisolid formulations at concentrations up to 30%
w/v [
32]. It is harmless to proteins when added to storing buffers at up to a 50% concentration, which is an important property when designing a formulation for protein drugs. HPMC was selected for its non-charged nature to avoid unwanted electrostatic interactions with the polyelectrolyte proteins. The 3%
w/v HPMC concentration was used because it allowed for a viscosity suitable for topical application (no dripping) even after autoclave sterilization, which is a necessary process for formulations to be administered to open ulcers.
The addition of glycerol was fundamental to slow down water evaporation from the formulation, a phenomenon which is deleterious for wet silica as it yields an irreversible syneresis that stops the diffusion of the embedded proteins. Silica syneresis is a phenomenon that occurs when no liquid phase is left to surround the silica polymer network. In this respect, glycerol prevents syneresis primarily by slowing down water loss, as shown in
Appendix A. In addition, being a small molecule, it was also free to diffuse within the silica polymer network, and its presence in the liquid phase retained by the silica network assisted in preventing the inorganic polymer from undergoing syneresis [
33].
The results in vivo clearly demonstrate the usefulness of this formulation approach. Notably, the administration of SB3 in either a buffer or metolose-G did not improve diabetic ulcer healing, indicating the importance of providing a constant supply of fresh bioactive agent at the site of action by means of a slow release.
It is also noteworthy that the use of the silica–metolose-G made it possible to highlight the usefulness of SB3 in the treatment of ulcers. This result—which potentially brings a novel pharmacological option for chronic ulcers, with great value for patients—could not have emerged in the absence of a suitable formulation.
A system to deliver SB3 and boost fibrogenesis and angiogenesis will represent an invaluable tool to improve wound healing. Our study, however, has some limitations. First, the treatments were applied acutely, whereas repeated administrations would probably improve the performances of the wet sol–gel silica slurry. Second, we tested a single dose, extrapolated by the experiments in vitro. Dose-ranging experiments, coupled with repeated administrations, would identify the best combinations for improving the efficacy of our treatment, and will be the subject of future investigations.
With respect to a potential clinical translation, one could say that the formulation was here tested only immediately after its preparation, and it is expected that, upon shelf storage, the protein would diffuse out from the wet silica gel to the HPMC hydrogel environment, so that the formulation would lose its SB3 slow-releasing and protecting features. However, it has to be noted that the protein release from wet silica almost stops when the gel is stored in a silicic acid-saturated solution [
18], a concentration that is reached quite fast when storing the wet gel in the minimum amount of buffer solution to keep it hydrated. As long as the hydration buffer is saturated with silicic acid, the protein will not diffuse out for the wet gel and the slurry will maintain its slow-releasing potential, which will be fully restored only upon dilution with the HPMC hydrogel. Therefore, a packaging solution capable of permitting the mixing of the two gels immediately prior to application should be devised to make this formulation approach translatable to the clinic.