Development and Validation of a Novel Tool for Assessing the Environmental Impact of 3D Printing Technologies: A Pharmaceutical Perspective
Abstract
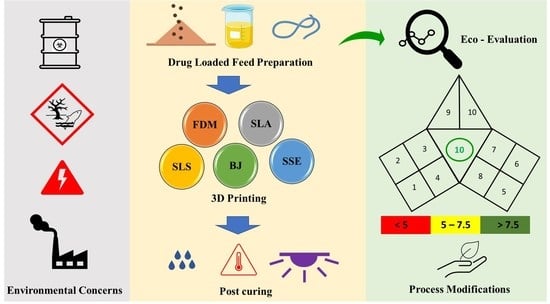
:1. Introduction
1.1. History of Environmental Awareness
1.2. Greenness Assessment Tools
1.3. 3DP of Pharmaceuticals
3DP Process and Methods
Fused Deposition Modeling
Stereolithography
Selective Laser Sintering
Semisolid Extrusion (SSE)

Binder Jetting (BJ)
1.4. Environmental Impacts of 3DP
2. Index of Greenness Assessment of Printed Pharmaceuticals (iGAPP)
2.1. Description of iGAPP Tool
2.1.1. Solvent Environmental Impact
2.1.2. Temperature Applied in the Feed Preparation Stage
2.1.3. Solvent Removal
2.1.4. Number of Active Constituents
2.1.5. Energy Consumed for 3DP
2.1.6. Printing Temperature
2.1.7. Printing Time
2.1.8. Waste Treatment
2.1.9. Post Curing Process
2.1.10. Time of Post-Curing Process
2.2. Validation of the iGAPP Tool
3. Limitations and Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kovalenko, K.; Kovalenko, N. Ecological problem of modernity as a global problem of humanity. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018. [Google Scholar]
- Linthorst, J. An overview: Origins and development of green chemistry. Found. Chem. 2010, 12, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Wardencki, W.; Curyło, J.; Namiesśnik, J. Green Chemistry—Current and Future Issues. Pol. J. Environ. Stud. 2005, 14, 389–395. [Google Scholar]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.L.; Smith, R.L.; Poliakoff, M. Principles of green chemistry: Productively. Green Chem. 2005, 7, 761–762. [Google Scholar] [CrossRef]
- Cue, B.W.; Zhang, J. Green process chemistry in the pharmaceutical industry. Green Chem. Lett. Rev. 2009, 2, 193–211. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef] [Green Version]
- Belkhir, L.; Elmeligi, A. Carbon footprint of the global pharmaceutical industry and relative impact of its major players. J. Clean. Prod. 2019, 214, 185–194. [Google Scholar] [CrossRef]
- Editors, P.T. Pharma Companies Join the American Business Act on Climate Pledge. 2015. Available online: https://www.pharmtech.com/view/pharma-companies-join-american-business-act-climate-pledge (accessed on 3 October 2021).
- Prat, D.; Hayler, J.; Wells, A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Andrews, I.; Cui, J.; DaSilva, J.; Dudin, L.; Dunn, P.; Hayler, J.; Hinkley, B.; Hughes, D.; Kaptein, B.; Kolis, S. Green chemistry articles of interest to the pharmaceutical industry. Org. Process Res. Dev. 2009, 13, 397–408. [Google Scholar] [CrossRef]
- Finnveden, G.; Hauschild, M.Z.; Ekvall, T.; Guinée, J.; Heijungs, R.; Hellweg, S.; Koehler, A.; Pennington, D.; Suh, S. Recent developments in life cycle assessment. J. Environ. Manag. 2009, 91, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Shuaib, M.; Haleem, A.; Kumar, S.; Javaid, M. Impact of 3D Printing on the environment: A literature-based study. Sustain. Oper. Comput. 2021, 2, 57–63. [Google Scholar] [CrossRef]
- Graedel, T.E. A structured approach to LCA improvement analysis. J. Ind. Ecol. 1999, 3, 85–93. [Google Scholar] [CrossRef]
- Toro, J.; Requena, I.; Duarte, O.; Zamorano, M. A qualitative method proposal to improve environmental impact assessment. Environ. Impact Assess. Rev. 2013, 43, 9–20. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Youssef, S.H.; Afinjuomo, F.; Song, Y.; Garg, S. Development of a novel chromatographic method for concurrent determination of 5-fluorouracil and cisplatin: Validation, greenness evaluation, and application on drug-eluting film. Microchem. J. 2021, 168, 106510. [Google Scholar] [CrossRef]
- El-Naem, O.A.; Saleh, S.S. Eco-friendly UPLC-MS/MS analysis of possible add-on therapy for COVID-19 in human plasma: Insights of greenness assessment. Microchem. J. 2021, 166, 106234. [Google Scholar] [CrossRef] [PubMed]
- Vállez-Gomis, V.; Peris-Pastor, G.; Benedé, J.L.; Chisvert, A.; Salvador, A. Green determination of eight water-soluble B vitamins in cosmetic products by liquid chromatography with ultraviolet detection. J. Pharm. Biomed. Anal. 2021, 205, 114308. [Google Scholar] [CrossRef] [PubMed]
- Keith, L.H.; Gron, L.U.; Young, J.L. Green analytical methodologies. Chem. Rev. 2007, 107, 2695–2708. [Google Scholar] [CrossRef] [PubMed]
- Julee Lynn Driver, D.R. Green assessment of chemical methods. In Proceedings of the 13th Green Chemistry Conference, College Park, MD, USA, 23–25 June 2009. [Google Scholar]
- Hartman, R.; Helmy, R.; Al-Sayah, M.; Welch, C.J. Analytical Method Volume Intensity (AMVI): A green chemistry metric for HPLC methodology in the pharmaceutical industry. Green Chem. 2011, 13, 934–939. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Gamal, M.; Naguib, I.A.; Panda, D.S.; Abdallah, F.F. Comparative study of four greenness assessment tools for selection of greenest analytical method for assay of hyoscine N-butyl bromide. Anal. Methods 2021, 13, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ji, Z.; Leu, M.C.; Caudill, R. Environmental performance analysis of solid freedom fabrication processes. In Proceedings of the 1999 IEEE International Symposium on Electronics and the Environment (Cat. No. 99CH36357), Danvers, MA, USA, 13 May 1999; pp. 1–6. [Google Scholar]
- Kong, Y.L.; Zou, X.; McCandler, C.A.; Kirtane, A.R.; Ning, S.; Zhou, J.; Abid, A.; Jafari, M.; Rogner, J.; Minahan, D. 3D—Printed gastric resident electronics. Adv. Mater. Technol. 2019, 4, 1800490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capel, A.J.; Rimington, R.P.; Lewis, M.P.; Christie, S.D. 3D printing for chemical, pharmaceutical and biological applications. Nat. Rev. Chem. 2018, 2, 422–436. [Google Scholar] [CrossRef]
- Park, B.J.; Choi, H.J.; Moon, S.J.; Kim, S.J.; Bajracharya, R.; Min, J.Y.; Han, H.-K. Pharmaceutical applications of 3D printing technology: Current understanding and future perspectives. J. Pharm. Investig. 2019, 49, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Gioumouxouzis, C.I.; Karavasili, C.; Fatouros, D.G. Recent advances in pharmaceutical dosage forms and devices using additive manufacturing technologies. Drug Discov. Today 2019, 24, 636–643. [Google Scholar] [CrossRef] [PubMed]
- McCue, T. Significant 3D Printing Forecast Surges to $35.6 Billion. Forbes. 2019. Available online: https://www.forbes.com/sites/tjmccue/2019/03/27/wohlers-report-2019-forecasts-35-6-billion-in-3d-printing-industry-growth-by-2024/ (accessed on 2 April 2021).
- Parhi, R. A review of three-dimensional printing for pharmaceutical applications: Quality control, risk assessment and future perspectives. J. Drug Deliv. Sci. Technol. 2021, 64, 102571. [Google Scholar] [CrossRef]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D printing in pharmaceutical and medical applications–recent achievements and challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Trivedi, M.; Jee, J.; Silva, S.; Blomgren, C.; Pontinha, V.M.; Dixon, D.L.; Van Tassel, B.; Bortner, M.J.; Williams, C.; Gilmer, E. Additive manufacturing of pharmaceuticals for precision medicine applications: A review of the promises and perils in implementation. Addit. Manuf. 2018, 23, 319–328. [Google Scholar] [CrossRef]
- Reddy, R.D.P.; Sharma, V. Additive manufacturing in drug delivery applications: A review. Int. J. Pharm. 2020, 589, 119820. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced pharmaceutical applications of hot-melt extrusion coupled with fused deposition modelling (FDM) 3D printing for personalised drug delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gioumouxouzis, C.I.; Baklavaridis, A.; Katsamenis, O.L.; Markopoulou, C.K.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. A 3D printed bilayer oral solid dosage form combining metformin for prolonged and glimepiride for immediate drug delivery. Eur. J. Pharm. Sci. 2018, 120, 40–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, R.; Chaves, P.; Goyanes, A.; Vukosavljevic, B.; Buanz, A.; Windbergs, M.; Basit, A.; Gaisford, S. 3D printed tablets loaded with polymeric nanocapsules: An innovative approach to produce customized drug delivery systems. Int. J. Pharm. 2017, 528, 268–279. [Google Scholar] [CrossRef]
- Ibrahim, M.; Barnes, M.; McMillin, R.; Cook, D.W.; Smith, S.; Halquist, M.; Wijesinghe, D.; Roper, T.D. 3D printing of metformin HCl PVA tablets by fused deposition modeling: Drug loading, tablet design, and dissolution studies. Aaps Pharmscitech 2019, 20, 195. [Google Scholar] [CrossRef]
- Ayyoubi, S.; Cerda, J.R.; Fernández-García, R.; Knief, P.; Lalatsa, A.; Healy, A.M.; Serrano, D.R. 3D printed spherical mini-tablets: Geometry versus composition effects in controlling dissolution from personalised solid dosage forms. Int. J. Pharm. 2021, 597, 120336. [Google Scholar] [CrossRef] [PubMed]
- Cerda, J.R.; Arifi, T.; Ayyoubi, S.; Knief, P.; Ballesteros, M.P.; Keeble, W.; Barbu, E.; Healy, A.M.; Lalatsa, A.; Serrano, D.R. Personalised 3D printed medicines: Optimising material properties for successful passive diffusion loading of filaments for fused deposition modelling of solid dosage forms. Pharmaceutics 2020, 12, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagishi, K.; Umezu, S. Development of the improving process for the 3D printed structure. Sci. Rep. 2017, 7, 39852. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Bártolo, P.J. 3D photo-fabrication for tissue engineering and drug delivery. Engineering 2015, 1, 90–112. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.; Pan, H.; Su, Y.; Fang, D.; Qiao, S.; Ding, P.; Pan, W. Opportunities and challenges of three-dimensional printing technology in pharmaceutical formulation development. Acta Pharm. Sin. B 2021, 11, 2488–2504. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giri, B.R.; Song, E.S.; Kwon, J.; Lee, J.-H.; Park, J.-B.; Kim, D.W. Fabrication of intragastric floating, controlled release 3D printed theophylline tablets using hot-melt extrusion and fused deposition modeling. Pharmaceutics 2020, 12, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Aita, I.; Breitkreutz, J.; Quodbach, J. On-demand manufacturing of immediate release levetiracetam tablets using pressure-assisted microsyringe printing. Eur. J. Pharm. Biopharm. 2019, 134, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zema, L.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Three-Dimensional Printing of Medicinal Products and the Challenge of Personalized Therapy. J. Pharm. Sci. 2017, 106, 1697–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdella, S.; Youssef, S.H.; Afinjuomo, F.; Song, Y.; Fouladian, P.; Upton, R.; Garg, S. 3D Printing of Thermo-Sensitive Drugs. Pharmaceutics 2021, 13, 1524. [Google Scholar] [CrossRef] [PubMed]
- Ghobadian, A.; Talavera, I.; Bhattacharya, A.; Kumar, V.; Garza-Reyes, J.A.; O’regan, N. Examining legitimatisation of additive manufacturing in the interplay between innovation, lean manufacturing and sustainability. Int. J. Prod. Econ. 2020, 219, 457–468. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Ginn, M.; Rastogi, V. A review of 3D printing techniques for environmental applications. Curr. Opin. Chem. Eng. 2020, 28, 173–178. [Google Scholar] [CrossRef]
- Gebler, M.; Uiterkamp, A.J.S.; Visser, C. A global sustainability perspective on 3D printing technologies. Energy Policy 2014, 74, 158–167. [Google Scholar] [CrossRef]
- Chan, F.L.; Hon, C.-Y.; Tarlo, S.M.; Rajaram, N.; House, R. Emissions and health risks from the use of 3D printers in an occupational setting. J. Toxicol. Environ. Health Part A 2020, 83, 279–287. [Google Scholar] [CrossRef]
- Jawaid, M.; Thariq, M.; Saba, N. Durability and Life Prediction in Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Khosravani, M.R.; Reinicke, T. On the environmental impacts of 3D printing technology. Appl. Mater. Today 2020, 20, 100689. [Google Scholar] [CrossRef]
- Gupta, N.; Weber, C.; Newsome, S. Additive Manufacturing: Status and Opportunities; Science and Technology Policy Institute: Washington, DC, USA, 2012. [Google Scholar]
- Mani, M.; Lyons, K.W.; Gupta, S.K. Sustainability Characterization for Additive Manufacturing. J. Res. Natl. Inst. Stand Technol. 2014, 119, 419–428. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Principles of green chemistry. In Green Chemistry Theory and Practice; Oxford University Press: New York, NY, USA, 1998; p. 29. [Google Scholar]
- Henderson, R.K.; Jiménez-González, C.; Constable, D.J.; Alston, S.R.; Inglis, G.G.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide–embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Chaudhari, V.S.; Malakar, T.K.; Murty, U.S.; Banerjee, S. Extruded filaments derived 3D printed medicated skin patch to mitigate destructive pulmonary tuberculosis: Design to delivery. Expert. Opin. Drug Deliv. 2021, 18, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Song, Y.; Fouladian, P.; Arafat, M.; Chung, R.; Kohlhagen, J.; Garg, S. Three-dimensional printing of curcumin-loaded biodegradable and flexible scaffold for intracranial therapy of glioblastoma multiforme. Pharmaceutics 2021, 13, 471. [Google Scholar] [CrossRef]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.-W.; Ahmed, W.; Arafat, B. Emergence of 3D printed dosage forms: Opportunities and challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A. Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation. Green Chem. 2008, 10, 31–36. [Google Scholar] [CrossRef]
- Prat, D.; Pardigon, O.; Flemming, H.-W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P. Sanofi’s solvent selection guide: A step toward more sustainable processes. Org. Process. Res. Dev. 2013, 17, 1517–1525. [Google Scholar] [CrossRef]
- Tagami, T.; Ando, M.; Nagata, N.; Goto, E.; Yoshimura, N.; Takeuchi, T.; Noda, T.; Ozeki, T. Fabrication of naftopidil-loaded tablets using a semisolid extrusion-type 3D printer and the characteristics of the printed hydrogel and resulting tablets. J. Pharm. Sci. 2019, 108, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allan, G.M.; Arroll, B. Prevention and treatment of the common cold: Making sense of the evidence. Cmaj 2014, 186, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Crooke, M. New Zealand cardiovascular guidelines: Best practice evidence-based guideline: The assessment and management of cardiovascular risk December 2003. Clin. Biochem. Rev. 2007, 28, 19. [Google Scholar] [PubMed]
- Holmberg, K.; Erdemir, A. Global impact of friction on energy consumption, economy and environment. Fme Trans 2015, 43, 181–185. [Google Scholar]
- Xu, X.; Meteyer, S.; Perry, N.; Zhao, Y.F. Energy consumption model of Binder-jetting additive manufacturing processes. Int. J. Prod. Res. 2015, 53, 7005–7015. [Google Scholar] [CrossRef]
- Kuźmińska, M.; Pereira, B.C.; Habashy, R.; Peak, M.; Isreb, M.; Gough, T.D.; Isreb, A.; Alhnan, M.A. Solvent-free temperature-facilitated direct extrusion 3D printing for pharmaceuticals. Int. J. Pharm. 2021, 598, 120305. [Google Scholar] [CrossRef]
- Agrawal, A.; Gupta, A.K. 3D Printing Technology in Pharmaceuticals and Biomedical: A Review. J. Drug Deliv. Ther. 2019, 9, 1–4. [Google Scholar]
- Jiang, J.; Xu, X.; Stringer, J. Support structures for additive manufacturing: A review. J. Manuf. Mater. Processing 2018, 2, 64. [Google Scholar] [CrossRef] [Green Version]
- Gil Olano, L.D. Structure-Property Relationship of Photo-Curable Resins for 3D Printing. 2020. Available online: https://bibliotecadigital.udea.edu.co/bitstream/10495/16217/7/GilLeon_2020_PhotocurableResinsPrinting.pdf (accessed on 15 January 2022).
- Schmitz, A. Effect of Three-Dimensional Printing with Nanotubes on Impact and Fatigue Resistance. J. Eng. Mater. Technol. 2020, 142, 024501. [Google Scholar] [CrossRef]
- Suwanprateeb, J.; Suwanpreuk, W. Development of translucent and strong three dimensional printing models. Rapid Prototyp. J. 2009, 15, 1355–2546. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Li, S.W.; Kowsari, K.; Shetty, A.; Sorrells, L.; Sen, K.; Nagapudi, K.; Chaudhuri, B.; Ma, A.W. Binder-jet 3D printing of indomethacin-laden pharmaceutical dosage forms. J. Pharm. Sci. 2020, 109, 3054–3063. [Google Scholar] [CrossRef]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Robles-Martinez, P.; Madla, C.M.; Joubert, F.; Goyanes, A.; Basit, A.W.; Gaisford, S. Stereolithography (SLA) 3D printing of an antihypertensive polyprintlet: Case study of an unexpected photopolymer-drug reaction. Addit. Manuf. 2020, 33, 101071. [Google Scholar] [CrossRef]
- Allahham, N.; Fina, F.; Marcuta, C.; Kraschew, L.; Mohr, W.; Gaisford, S.; Basit, A.W.; Goyanes, A. Selective laser sintering 3D printing of orally disintegrating printlets containing ondansetron. Pharmaceutics 2020, 12, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johannesson, J.; Khan, J.; Hubert, M.; Teleki, A.; Bergström, C.A. 3D-printing of solid lipid tablets from emulsion gels. Int. J. Pharm. 2021, 597, 120304. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Mancinelli, C.; Mancuso, E.; García-Romero, I.; Gilmore, B.F.; Casettari, L.; Larrañeta, E.; Lamprou, D.A. 3D printing of drug-loaded thermoplastic polyurethane meshes: A potential material for soft tissue reinforcement in vaginal surgery. Pharmaceutics 2020, 12, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempin, W.; Franz, C.; Koster, L.-C.; Schneider, F.; Bogdahn, M.; Weitschies, W.; Seidlitz, A. Assessment of different polymers and drug loads for fused deposition modeling of drug loaded implants. Eur. J. Pharm. Biopharm. 2017, 115, 84–93. [Google Scholar] [CrossRef]
- Yi, H.-G.; Choi, Y.-J.; Kang, K.S.; Hong, J.M.; Pati, R.G.; Park, M.N.; Shim, I.K.; Lee, C.M.; Kim, S.C.; Cho, D.-W. A 3D-printed local drug delivery patch for pancreatic cancer growth suppression. J. Control Release 2016, 238, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, M.; Nikjoo, D.; Gustafsson, T.; Gaisford, S.; Basit, A. Pressure-assisted microsyringe 3D printing of oral films based on pullulan and hydroxypropyl methylcellulose. Int. J. Pharm. 2021, 595, 120197. [Google Scholar] [CrossRef] [PubMed]




| P | Prevent waste |
| R | Renewable material |
| O | Omit derivatization steps |
| D | Degradable chemical products |
| U | Use safe synthetic methods |
| C | Catalytic reagents |
| T | Temperature, pressure ambient |
| I | In-process monitoring |
| V | Very few auxiliary substances |
| E | E-factor, maximise feed in product |
| L | Low toxicity of chemical products |
| Y | Yes, it is safe |
 | ||||
|---|---|---|---|---|
| Green (Score = 100%) | Yellow (Score = 50%) | Red (Score = 0%) | ||
| (a) Pre-processing parameters | ||||
| 1 | Solvent environmental impact * (1 point) | Environmental impact score: ≥8 | Environmental impact score: 4–7 | Environmental impact score: ≤3 |
| 2 | Temperature (°C) (1 point) | <30 | 30–60 | >60 |
| 3 | Solvent removal (1 point) | No solvent removal required | Evaporation at room temperature | Evaporation at temperature >25 |
| 4 | No. active constituents (1 point) | >2 | 2 | 1 |
| (b) Printing process | ||||
| 5 | Energy consumption (2 points) | BJ SSE (<100 kPa) | SLA SLS SSE (100–500 kPa) | FDM SSE (>500 kPa) |
| 6 | Temperature (°C) (1 point) | Room temperature | 26–10 | >110 |
| 7 | Printing time per product (min) (1 point) | <2.5 | 2.5–10 | >10 |
| 8 | Waste treatment (1 point) | No waste | Waste is recycled | Waste is disposed |
| (c) Post curing | ||||
| 9 | Post curing process (0.5 point) | No post-curing/ non-energy consuming process | Drying at temperature <60 °C | Higher energy-consuming post-curing process |
| 10 | Time of post-curing process (hours) (0.5 point) | No post-curing | <1 | >1 |
| (d) Total score | ||||
| >7.5 | 5–7.5 | <5 | ||
| BJ [79] | FDM [80] | SLA [81] | SLS [82] | SSE [83] | |
|---|---|---|---|---|---|
| iGAPP pictogram |  |  |  |  |  |
| Solvent environmental impact | Water: 10 (1) | No solvent (1) | No solvent (1) | Ethanol: 8 (1) | Propanol: 7 (0.5) |
| Temperature (°C) | Room temperature (1) | 80–110 (0) | Room temperature (1) | Room temperature (1) | 70 (0) |
| Solvent removal | No solvent removal (1) | No solvent removal (1) | No solvent removal (1) | 40 °C (0) | No solvent removal (1) |
| No. active constituents | 1 (0) | 1 (0) | 4 (1) | 1 (0) | 1 (0) |
| Energy consumption | BJ (2) | FDM (0) | SLA (1) | SLS (1) | SSE (55–65 kPa) (2) |
| Temperature (°C) | Room temperature (1) | 180–190 (0) | Room temperature (1) | 80–100 (0.5) | Room temperature (1) |
| Printing time per product (min) | 2.5 (0.5) | <2.5 min (1) | 2.5–10 (0.5) | <2.5 min (1) | 11–14 min (0) |
| Waste treatment | Recycle (0.5) | No waste (1) | Waste is disposed (0) | Recycle (0.5) | No waste (1) |
| Post curing | Drying 40 °C (0.25) | No post curing (0.5) | non-energy consuming process (0.5) | non-energy consuming process (0.5) | Drying at room temperature (0.25) |
| Time of post-curing process (hours) | Washing + Drying (>1 h) (0) | No post curing (0.5) | Washing (<1 h) (0.25) | Powder removal (<1 h) (0.25) | Drying (>1 h) (0) |
| FDM 1 [84] | FDM 2 [85] | |
|---|---|---|
| iGAPP pictogram |  |  |
| Solvent environmental impact | No solvent (1) | Environmental impact score: 6 (0.5) |
| Temperature (°C) | 170–200 (0) | 140 (0) |
| Solvent removal | No solvent removal (1) | Solvent removal (0.5) |
| No. active constituents | 1 (0) | 1 (0) |
| Energy consumption | FDM (0) | FDM (0) |
| Temperature (°C) | 195–208 (0) | 164 (0) |
| Printing time per product (min) | <2.5 min (1) | 2.5–10 (0.5) |
| Waste treatment | No waste (1) | No waste (1) |
| Post curing | No post-curing (0.5) | No post-curing (0.5) |
| Time of post-curing process (hours) | No post-curing (0.5) | No post-curing (0.5) |
| SSE 1 [86] | SSE 2 [87] | |
|---|---|---|
| iGAPP pictogram |  |  |
| Solvent environmental impact | No solvent (1) | Environmental impact score: 10 (1) |
| Temperature (°C) | 140 (0) | 70 (0) |
| Solvent removal | No solvent removal (1) | No solvent removal (1) |
| No. active constituents | 1 (0) | 1 (0) |
| Energy consumption | SSE (600 kPa) (0) | SSE (65 kPa) (2) |
| Temperature (°C) | 140 (0) | Room temperature (1) |
| Printing time per product (min) | <2.5 min (1) | <2.5 min (1) |
| Waste treatment | No waste (1) | No waste (1) |
| Post curing | No post-curing (0.5) | Drying at room temperature (0.5) |
| Time of post-curing process (hours) | No post-curing (0.5) | Drying (>1 h) (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, S.H.; Abdella, S.; Garg, S. Development and Validation of a Novel Tool for Assessing the Environmental Impact of 3D Printing Technologies: A Pharmaceutical Perspective. Pharmaceutics 2022, 14, 933. https://doi.org/10.3390/pharmaceutics14050933
Youssef SH, Abdella S, Garg S. Development and Validation of a Novel Tool for Assessing the Environmental Impact of 3D Printing Technologies: A Pharmaceutical Perspective. Pharmaceutics. 2022; 14(5):933. https://doi.org/10.3390/pharmaceutics14050933
Chicago/Turabian StyleYoussef, Souha H., Sadikalmahdi Abdella, and Sanjay Garg. 2022. "Development and Validation of a Novel Tool for Assessing the Environmental Impact of 3D Printing Technologies: A Pharmaceutical Perspective" Pharmaceutics 14, no. 5: 933. https://doi.org/10.3390/pharmaceutics14050933