Citrus Flavanone Narirutin, In Vitro and In Silico Mechanistic Antidiabetic Potential
Abstract
:1. Introduction
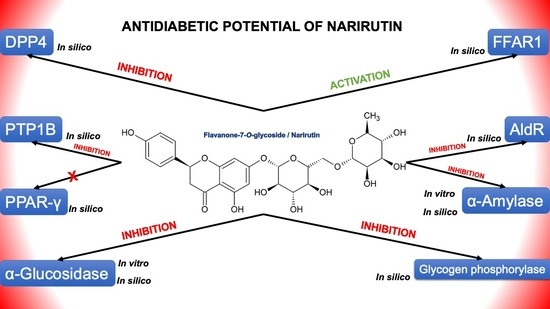
2. Results and Discussion
2.1. Molecular Docking
2.1.1. PTP1B
2.1.2. DPP4
2.1.3. FFAR1
2.1.4. Alpha-Amylase
2.1.5. PPARγ
2.1.6. Alpha-Glucosidase
2.1.7. Aldose Reductase
2.1.8. Glycogen Phosphorylase
2.2. In Vitro Assays
2.2.1. Alpha-Amylase Inhibitory Effect
2.2.2. Alpha-Glucosidase Inhibitory Effect
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Molecular Docking
3.2.1. Preparation of the Ligand
3.2.2. Preparation of the Receptors
3.2.3. Simulation
3.3. Narirutin In-Vitro Inhibition Potential on Digestive Enzymes
3.3.1. Alpha-Amylase Inhibition Assay
3.3.2. Alpha-Glucosidase Inhibitory Assay
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; IDF: Brussels, Belgium, 2017. [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2015; ISBN 978-2-930229-81-2. [Google Scholar]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF Diabetes Atlas: Global Estimates of the Prevalence of Diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef]
- IDF. IDF Diabetes Atlas, 6th ed.; International Diabetes Federation: Brussels, Belgium, 2013. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. Economic Costs of Diabetes in the US in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Ding, L.; Liu, J.; Su, L.; Dong, J.; Liao, L. Efficacy and Short-Term Side Effects of Sitagliptin, Vildagliptin and Saxagliptin in Chinese Diabetes: A Randomized Clinical Trial. Endocr. Connect. 2019, 8, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Mechchate, H.; Es-safi, I.; Mohamed Al kamaly, O.; Bousta, D. Insight into Gentisic Acid Antidiabetic Potential Using In Vitro and In Silico Approaches. Molecules 2021, 26, 1932. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Kader, F.B.; Arman, M.; Ahmed, S.; Lyzu, C.; Sakib, S.A.; Tanzil, S.M.; Zim, A.I.U.; Imran, M.A.S.; Venneri, T. Pharmacological Insights and Prediction of Lead Bioactive Isolates of Dita Bark through Experimental and Computer-Aided Mechanism. Biomed. Pharmacother. 2020, 131, 110774. [Google Scholar] [CrossRef] [PubMed]
- Es-safi, I.; Mechchate, H.; Amaghnouje, A.; Elbouzidi, A.; Bouhrim, M.; Bencheikh, N.; Hano, C.; Bousta, D. Assessment of Antidepressant-Like, Anxiolytic Effects and Impact on Memory of Pimpinella Anisum L. Total Extract on Swiss Albino Mice. Plants 2021, 10, 1573. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Mahmoodi, M.; Munir, A.; Zahra, S.A.; Shahbaz, A.; Shaukat, M.; Kanwal, S.; Uddin, S. Phytogenic Synthesis of Nickel Oxide Nanoparticles (NiO) Using Fresh Leaves Extract of Rhamnus Triquetra (Wall.) and Investigation of Its Multiple in Vitro Biological Potentials. Biomedicines 2020, 8, 117. [Google Scholar] [CrossRef]
- Wirnitzer, K.C. Vegan nutrition: Latest boom in health and exercise. In Therapeutic, Probiotic, and Unconventional Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 387–453. [Google Scholar]
- Alissa, E.M.; Ferns, G.A. Dietary Fruits and Vegetables and Cardiovascular Diseases Risk. Crit. Rev. Food Sci. Nutr. 2017, 57, 1950–1962. [Google Scholar] [CrossRef] [Green Version]
- Mechchate, H.; Costa de Oliveira, R.; Es-safi, I.; Vasconcelos Mourão, E.M.; Bouhrim, M.; Kyrylchuk, A.; Soares Pontes, G.; Bousta, D.; Grafov, A. Antileukemic Activity and Molecular Docking Study of a Polyphenolic Extract from Coriander Seeds. Pharmaceuticals 2021, 14, 770. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Büsselberg Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodowska, K.M. Natural Flavonoids: Classification, Potential Role, and Application of Flavonoid Analogues. Eur. J. Biol. Res. 2017, 7, 108–123. [Google Scholar]
- Ghasemzadeh, A. Flavonoids and Phenolic Acids: Role and Biochemical Activity in Plants and Human. J. Med. Plants Res. 2011, 5. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Mai, Y.; Li, H.; Wang, Z.; Xu, J.; He, X. Health Benefits of the Flavonoids from Onion: Constituents and Their Pronounced Antioxidant and Anti-Neuroinflammatory Capacities. J. Agric. Food Chem. 2020, 68, 799–807. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, Flavones, Flavanones, and Human Health: Epidemiological Evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus Phytochemical with Health-promoting Properties. BioFactors 2017, 43, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Scafuri, B.; Bontempo, P.; Altucci, L.; De Masi, L.; Facchiano, A. Molecular Docking Simulations on Histone Deacetylases (HDAC)-1 and -2 to Investigate the Flavone Binding. Biomedicines 2020, 8, 568. [Google Scholar] [CrossRef]
- Velmurugan, B.K.; Lin, J.-T.; Mahalakshmi, B.; Chuang, Y.-C.; Lin, C.-C.; Lo, Y.-S.; Hsieh, M.-J.; Chen, M.-K. Luteolin-7-O-Glucoside Inhibits Oral Cancer Cell Migration and Invasion by Regulating Matrix Metalloproteinase-2 Expression and Extracellular Signal-Regulated Kinase Pathway. Biomolecules 2020, 10, 502. [Google Scholar] [CrossRef]
- Jia, Z.; Barford, D.; Flint, A.; Tonks, N. Structural Basis for Phosphotyrosine Peptide Recognition by Protein Tyrosine Phosphatase 1B. Science 1995, 268, 1754–1758. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.O.; Ermolieff, J.; Jirousek, M.R. Protein Tyrosine Phosphatase 1B Inhibitors for Diabetes. Nat. Rev. Drug Discov. 2002, 1, 696–709. [Google Scholar] [CrossRef]
- Wiesmann, C.; Barr, K.J.; Kung, J.; Zhu, J.; Erlanson, D.A.; Shen, W.; Fahr, B.J.; Zhong, M.; Taylor, L.; Randal, M.; et al. Allosteric Inhibition of Protein Tyrosine Phosphatase 1B. Nat. Struct. Mol. Biol. 2004, 11, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Bjelke, J.R.; Christensen, J.; Branner, S.; Wagtmann, N.; Olsen, C.; Kanstrup, A.B.; Rasmussen, H.B. Tyrosine 547 Constitutes an Essential Part of the Catalytic Mechanism of Dipeptidyl Peptidase IV. J. Biol. Chem. 2004, 279, 34691–34697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, C.-H.; Tsai, C.-H.; Lin, C.-H.; Chou, C.-Y.; Chen, X. Identification of Hydrophobic Residues Critical for DPP-IV Dimerization †. Biochemistry 2006, 45, 7006–7012. [Google Scholar] [CrossRef]
- Morgan, N.G.; Dhayal, S. G-Protein Coupled Receptors Mediating Long Chain Fatty Acid Signalling in the Pancreatic Beta-Cell. Biochem. Pharmacol. 2009, 78, 1419–1427. [Google Scholar] [CrossRef] [Green Version]
- Sum, C.S.; Tikhonova, I.G.; Costanzi, S.; Gershengorn, M.C. Two Arginine-Glutamate Ionic Locks Near the Extracellular Surface of FFAR1 Gate Receptor Activation. J. Biol. Chem. 2009, 284, 3529–3536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sum, C.S.; Tikhonova, I.G.; Neumann, S.; Engel, S.; Raaka, B.M.; Costanzi, S.; Gershengorn, M.C. Identification of Residues Important for Agonist Recognition and Activation in GPR40. J. Biol. Chem. 2007, 282, 29248–29255. [Google Scholar] [CrossRef] [Green Version]
- Hsiu, J.; Fischer, E.H.; Stein, E.A. Alpha-amylases as calcium-metalloenzymes. II. Calcium and the catalytic activity. Biochemistry 1964, 3, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ragunath, C.; Manuel, S.G.A.; Venkataraman, V.; Sait, H.B.R.; Kasinathan, C.; Ramasubbu, N. Probing the Role of Aromatic Residues at the Secondary Saccharide-Binding Sites of Human Salivary α-Amylase in Substrate Hydrolysis and Bacterial Binding. J. Mol. Biol. 2008, 384, 1232–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasubbu, N.; Ragunath, C.; Sundar, K.; Mishra, P.J.; Gyémánt, G.; Kandra, L. Structure-Function Relationships in Human Salivary α-Amylase: Role of Aromatic Residues. Biol.-Sect. Cell. Mol. Biol. 2005, 60, 47–56. [Google Scholar]
- Ramasubbu, N.; Ragunath, C.; Mishra, P.J.; Thomas, L.M.; Gyemant, G.; Kandra, L. Human Salivary Alpha-Amylase Trp58 Situated at Subsite -2 Is Critical for Enzyme Activity. Eur. J. Biochem. 2004, 271, 2517–2529. [Google Scholar] [CrossRef]
- Pochetti, G.; Godio, C.; Mitro, N.; Caruso, D.; Galmozzi, A.; Scurati, S.; Loiodice, F.; Fracchiolla, G.; Tortorella, P.; Laghezza, A.; et al. Insights into the Mechanism of Partial Agonism: Crystal Structures Of The Peroxisome Proliferator-Activated Receptor Γ Ligand-Binding Domain In The Complex With Two Enantiomeric Ligands. J. Biol. Chem. 2007, 282, 17314–17324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, M.T.; ElGamal, A.A.; Al-Massarani, S.; Razmovski-Naumovski, V.; Alqahtani, M.S.; El Dib, R.A.; AlAjmi, M.F. Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia Oppositifolia. Biomolecules 2019, 10, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermans, M.M.; Kroos, M.A.; van Beeumen, J.; Oostra, B.A.; Reuser, A.J. Human Lysosomal Alpha-Glucosidase. Characterization of the Catalytic Site. J. Biol. Chem. 1991, 266, 13507–13512. [Google Scholar] [CrossRef]
- Wilson, D.K.; Bohren, K.M.; Gabbay, K.H.; Quiocho, F.A. An Unlikely Sugar Substrate Site in the 1.65 A Structure of the Human Aldose Reductase Holoenzyme Implicated in Diabetic Complications. Science 1992, 257, 81–84. [Google Scholar] [CrossRef]
- Barford, D.; Johnson, L.N. The Allosteric Transition of Glycogen Phosphorylase. Nature 1989, 340, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, E.; Sprang, S.R.; Hamlin, R.; Xuong, N.; Fletterick, R. Domain Separation in the Activation of Glycogen Phosphorylase a. Science 1989, 245, 528–532. [Google Scholar] [CrossRef]
- Elchebly, M. Increased Insulin Sensitivity and Obesity Resistance in Mice Lacking the Protein Tyrosine Phosphatase-1B Gene. Science 1999, 283, 1544–1548. [Google Scholar] [CrossRef]
- Goldstein, B.J. Protein-Tyrosine Phosphatase 1B (PTP1B): A Novel Therapeutic Target for Type 2 Diabetes Mellitus, Obesity and Related States of Insulin Resistance. Curr. Drug Targets Immune Endocr. Metab. Disord. 2001, 1, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Ketsawatsomkron, P.; Stepp, D.W.; de Chantemèle, E.J.B. PTP1B in Obesity-Related Cardiovascular Function. In Protein Tyrosine Phosphatase Control of Metabolism; Bence, K.K., Ed.; Springer: New York, NY, USA, 2013; pp. 129–145. ISBN 978-1-4614-7855-3. [Google Scholar]
- Zhang, Z.-Y.; Lee, S.-Y. PTP1B Inhibitors as Potential Therapeutics in the Treatment of Type 2 Diabetes and Obesity. Expert Opin. Investig. Drugs 2003, 12, 223–233. [Google Scholar] [CrossRef]
- Matteucci, E.; Giampietro, O. Dipeptidyl Peptidase-4 (CD26): Knowing the Function before Inhibiting the Enzyme. Curr. Med. Chem. 2009, 16, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Jung, E.; Yoon, M.K.; Kwon, O.H.; Hwang, D.-M.; Kim, D.-W.; Kim, J.; Lee, S.-M.; Yim, H.J. Pharmacological Profiles of Gemigliptin (LC15-0444), a Novel Dipeptidyl Peptidase-4 Inhibitor, in Vitro and in Vivo. Eur. J. Pharmacol. 2016, 788, 54–64. [Google Scholar] [CrossRef]
- Abd El-Karim, S.S.; Anwar, M.M.; Syam, Y.M.; Nael, M.A.; Ali, H.F.; Motaleb, M.A. Rational Design and Synthesis of New Tetralin-Sulfonamide Derivatives as Potent Anti-Diabetics and DPP-4 Inhibitors: 2D & 3D QSAR, in Vivo Radiolabeling and Bio Distribution Studies. Bioorg. Chem. 2018, 81, 481–493. [Google Scholar] [CrossRef]
- Ren, X.-M.; Cao, L.-Y.; Zhang, J.; Qin, W.-P.; Yang, Y.; Wan, B.; Guo, L.-H. Investigation of the Binding Interaction of Fatty Acids with Human G Protein-Coupled Receptor 40 Using a Site-Specific Fluorescence Probe by Flow Cytometry. Biochemistry 2016, 55, 1989–1996. [Google Scholar] [CrossRef]
- Srivastava, A.; Yano, J.; Hirozane, Y.; Kefala, G.; Gruswitz, F.; Snell, G.; Lane, W.; Ivetac, A.; Aertgeerts, K.; Nguyen, J.; et al. High-Resolution Structure of the Human GPR40 Receptor Bound to Allosteric Agonist TAK-875. Nature 2014, 513, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Suvd, D.; Fujimoto, Z.; Takase, K.; Matsumura, M.; Mizuno, H. Crystal Structure of Bacillus Stearothermophilus Alpha-Amylase: Possible Factors Determining the Thermostability. J. Biochem. (Tokyo) 2001, 129, 461–468. [Google Scholar] [CrossRef]
- Aghajari, N.; Feller, G.; Gerday, C.; Haser, R. Crystal Structures of the Psychrophilic Alpha-Amylase from Alteromonas Haloplanctis in Its Native Form and Complexed with an Inhibitor. Protein Sci. Publ. Protein Soc. 1998, 7, 564–572. [Google Scholar] [CrossRef] [Green Version]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ Signaling and Metabolism: The Good, the Bad and the Future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Rees, W.D.; McNeil, C.J.; Maloney, C.A. The Roles of PPARs in the Fetal Origins of Metabolic Health and Disease. PPAR Res. 2008, 2008, 1–8. [Google Scholar] [CrossRef]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Antidiabetic Potential of Gallic Acid from Emblica Officinalis: Improved Glucose Transporters and Insulin Sensitivity through PPAR-γ and Akt Signaling. Phytomedicine 2020, 73, 152906. [Google Scholar] [CrossRef]
- Attjioui, M.; Ryan, S.; Ristic, A.K.; Higgins, T.; Goñi, O.; Gibney, E.R.; Tierney, J.; O’Connell, S. Comparison of Edible Brown Algae Extracts for the Inhibition of Intestinal Carbohydrate Digestive Enzymes Involved in Glucose Release from the Diet. J. Nutr. Sci. 2021, 10, e5. [Google Scholar] [CrossRef]
- Tang, W.H.; Martin, K.A.; Hwa, J. Aldose Reductase, Oxidative Stress, and Diabetic Mellitus. Front. Pharmacol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Heather, L.C.; Clarke, K. Metabolism, Hypoxia and the Diabetic Heart. J. Mol. Cell. Cardiol. 2011, 50, 598–605. [Google Scholar] [CrossRef]
- Livanova, N.B.; Chebotareva, N.A.; Eronina, T.B.; Kurganov, B.I. Pyridoxal 5′-Phosphate as a Catalytic and Conformational Cofactor of Muscle Glycogen Phosphorylase B. Biochem. Biokhimiia 2002, 67, 1089–1098. [Google Scholar] [CrossRef]
- Ashworth, W.B.; Davies, N.A.; Bogle, I.D.L. A Computational Model of Hepatic Energy Metabolism: Understanding Zonated Damage and Steatosis in NAFLD. PLoS Comput. Biol. 2016, 12, e1005105. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-Safi, I.; Bourhia, M.; Kyrylchuk, A.; El Moussaoui, A.; Conte, R.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Grafov, A.; et al. In-Vivo Antidiabetic Activity and In-Silico Mode of Action of LC/MS-MS Identified Flavonoids in Oleaster Leaves. Molecules 2020, 25, 5073. [Google Scholar] [CrossRef]
- Su, C.; Yang, C.; Gong, M.; Ke, Y.; Yuan, P.; Wang, X.; Li, M.; Zheng, X.; Feng, W. Antidiabetic Activity and Potential Mechanism of Amentoflavone in Diabetic Mice. Molecules 2019, 24, 2184. [Google Scholar] [CrossRef] [Green Version]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The Protein Data Bank. Acta Crystallogr. D Biol. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Mechchate, H.; Es-safi, I.; Louba, A.; Alqahtani, A.S.; Nasr, F.A.; Noman, O.M.; Farooq, M.; Alharbi, M.S.; Alqahtani, A.; Bari, A.; et al. In Vitro Alpha-Amylase and Alpha-Glucosidase Inhibitory Activity and In Vivo Antidiabetic Activity of Withania Frutescens L. Foliar Extract. Molecules 2021, 26, 293. [Google Scholar] [CrossRef]
- Pistia-Brueggeman, G.; Hollingsworth, R.I. A Preparation and Screening Strategy for Glycosidase Inhibitors. Tetrahedron 2001, 57, 8773–8778. [Google Scholar] [CrossRef]










| Receptor | Affinity (kcal/mol) | Active Site Described in Literature | Interaction Confirmed with the Active Site | H-Bonds |
|---|---|---|---|---|
| PTP1B | −8.5 | Trp179, Pro180, Asp181 [26], His214, Ser216, Ala217, Gly218, Ile219, Gly220, and Arg221 [27,28] and the active site on Cys 215 (catalytic loop) | Yes | Arg221, Arg24, Ser216 |
| DPP4 | −10.4 | DPP4 active site (α/β-hydrolase domain) is identified by the residues from 39 to 51 and from 501 to 706 [29,30]. | Yes | Tyr662, Ser209, Ser630, Arg125, His740, Trp629, |
| FFAR1 | −8.3 | Active site includes Arg183, Arg258 and Tyr2240 [31]. Binding pocket is on Glu172, Arg183, Ser187, Tyr240, Asn241, Asn244, Arg258 and Tyr91 [32,33]. | Yes | Tyr44 |
| Alpha amylase | −9.9 | Active site:Asp197, Glu233 and Asp300 and other important AA: Arg337, Arg195, Asn298, Phe265, Phe295, His201, Ala307, Gly306, Trp203, Trp284, Trp59, Tyr62, Trp58, His299 and His101 [34,35,36,37]. | Yes | Gly306, Asp197, Gln63, Trp69, Lys352, Asp352 |
| PPAR gamma | - | PPARγ ligand-binding domain: Ser289, His323, Tyr473, and His449 [38]. | No | |
| Alpha glucosidase | −8.7 | The amino acids involved in the α-Glucosidase activity are Asp404, Asp518, Arg600, Asp616, and His674 Trp376, Ile441, Trp516, Met519, Trp613, and Phe649 Leu405, Trp481, Asp645, and Arg672 [39,40]. | Yes | Asp616, Ala284, Arg281, Asp282, Ser523 |
| Aldose reductase | −9.3 | The active site is located in the barrel core clearly seen in the 3D structure [41] | Yes | Val47, Gln49, Lys21, Ser32 |
| Glycogen phosphorylase | −8.3 | Active site on amino acids 280–288 (The 280’s loop) [42,43]. | Yes | Asp283, Glu382, leu384 |
| Alpha-Amylase In Vitro IC50 (mg/mL) | Alpha-Glucosidase In Vitro IC50 (mg/mL) | Alpha-Amylase In Silico Affinity (kcal/mol) | Alpha-Glucosidase In Silico Affinity (kcal/mol) | |
|---|---|---|---|---|
| Narirutin | 0.0066 | 0.00091 | −9.9 | −8.7 |
| Acarbose | 1.012 | 0.00035 | −8.1 | −8.4 |
| Receptor | PID | Resolution (Å) | Classification |
|---|---|---|---|
| Protein tyrosine phosphatase 1B (PTP1B) | 1c83 | 1.80 | Hydrolase |
| Glycogen phosphorylase (GP) | 1l5q | 2.25 | Transferase |
| Free fatty acid receptor 1 (FFAR1) | 4phu | 2.33 | Hydrolase |
| Peroxisome proliferator-activated receptor gamma (PPAR gamma) | 5ycp | 2.00 | Transcription |
| Alpha-amylase (AAM) | 1smd | 1.60 | Hydrolase |
| Alpha-glucosidase (AGL) | 5nn5 | 2.00 | Hydrolase |
| Aldose reductase (AR) | 2hv5 | 1.59 | Oxidoreductase |
| dipeptidyl peptidase IV (DPP4) | 2p8s | 2.20 | Hydrolase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qurtam, A.A.; Mechchate, H.; Es-safi, I.; Al-zharani, M.; Nasr, F.A.; Noman, O.M.; Aleissa, M.; Imtara, H.; Aleissa, A.M.; Bouhrim, M.; et al. Citrus Flavanone Narirutin, In Vitro and In Silico Mechanistic Antidiabetic Potential. Pharmaceutics 2021, 13, 1818. https://doi.org/10.3390/pharmaceutics13111818
Qurtam AA, Mechchate H, Es-safi I, Al-zharani M, Nasr FA, Noman OM, Aleissa M, Imtara H, Aleissa AM, Bouhrim M, et al. Citrus Flavanone Narirutin, In Vitro and In Silico Mechanistic Antidiabetic Potential. Pharmaceutics. 2021; 13(11):1818. https://doi.org/10.3390/pharmaceutics13111818
Chicago/Turabian StyleQurtam, Ashraf Ahmed, Hamza Mechchate, Imane Es-safi, Mohammed Al-zharani, Fahd A. Nasr, Omar M. Noman, Mohammed Aleissa, Hamada Imtara, Abdulmalik M. Aleissa, Mohamed Bouhrim, and et al. 2021. "Citrus Flavanone Narirutin, In Vitro and In Silico Mechanistic Antidiabetic Potential" Pharmaceutics 13, no. 11: 1818. https://doi.org/10.3390/pharmaceutics13111818