Control Strategy for Process Development of High-Shear Wet Granulation and Roller Compaction to Prepare a Combination Drug Using Integrated Quality by Design
Abstract
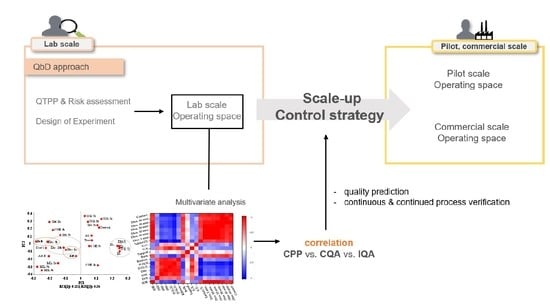
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design to Optimize the Manufacturing Process
2.2.1. Experimental Design of High-Shear Wet Granulation
2.2.2. Experimental Design of Roller Compaction
2.3. Measurement of IQAs and CQAs
2.3.1. Measurement of Intrinsic Dissolution Rate
2.3.2. Measurement of Granule Properties (Granule Size, Granule Density, Flowability, and Granule Strength)
2.3.3. Measurement of Swelling Property
2.3.4. Measurement of Weight Gain and Mass Loss
2.3.5. Measurement of Gel Strength
2.3.6. In Vitro Dissolution Test
2.3.7. Measurement of Contact Angle
2.4. HPLC Analysis Method
2.5. Multivariate Analysis
2.6. Initial Risk Assessment for the Manufacturing Process Development
3. Results and Discussion
3.1. Initial Risk Assessment for the Manufacturing Process Development
3.1.1. Initial Risk Assessment for High-Shear Wet Granulation
3.1.2. Initial Risk Assessment for Roller Compaction
3.2. Effect of CPPs on the IQAs and CQAs of High-Shear Wet Granulation
3.2.1. Effect of CPPs on the Intrinsic Dissolution Rate (q1)
3.2.2. Effect of CPPs on Granule Size (q2–q6)
3.2.3. Effect of CPPs on True Density, Bulk Density, Carr’s Index, and Angle of Repose (q7–q10)
3.2.4. Effect of CPPs on Granule Strength (q11)
3.2.5. Effect of CPPs on the Swelling Property (q12, q13, and q14)
3.2.6. Effect of CPPs on Weight Gain (q15, q16, and q17) and Mass Loss (q18, q19, and q20)
3.2.7. Effect of CPPs on Gel Strength (q21, q22, and q23)
3.2.8. Effect of CPPs on Dissolution (q24, q25, and q26)
3.2.9. Effect of CPPs on the Contact Angle (q27)
3.3. Effect of CPPs on the IQAs and CQAs of Roller Compaction
3.3.1. Effect of CPPs on the Intrinsic Dissolution Rate (d1)
3.3.2. Effect of CPPs on Granule Size (d2–d6)
3.3.3. Effect of CPPs on Ribbon Density, Bulk Density, and Tapped Density (d7, d8, and d9)
3.3.4. Effect of CPPs on Granule Strength and Granule Uniformity (d10 and d11)
3.3.5. Effect of CPPs on Tablet C.U. (d12)
3.3.6. Effect of CPPs on Dissolution (d13–d15)
3.3.7. Effect of CPPs on the Contact Angle (d16)
3.4. Design Spaces of Process Parameters
3.4.1. Design Space of High-Shear Wet Granulation
3.4.2. Design Space of Roller Compaction
3.5. Multivariate Analysis between IQAs and CQAs
3.5.1. Correlation between IQAs and CQAs of High Shear Wet Granulation Process
3.5.2. Correlation between the IQAs and CQAs of the Roller Compaction Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Björn, I.N.; Jansson, A.; Karlsson, M.; Folestad, S.; Rasmuson, A. Empirical to mechanistic modelling in high shear granulation. Chem. Eng. Sci. 2005, 60, 3795–3803. [Google Scholar] [CrossRef]
- Dumarey, M.; Talwar, S.; Yahyah, M.; Peterson, J. Empirical modelling to support scale up of primary pharmaceutical processes. In Computer Aided Chemical Engineerin; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 2241–2246. [Google Scholar]
- Lawrence, X.Y. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2008, 25, 781–791. [Google Scholar]
- Pandey, P.; Bharasdwaj, R.; Chen, X. Modeling of drug product manufacturing processes in the pharmaceutical industry. In Predictive Modeling of Pharmaceutical Unit Operations; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–13. [Google Scholar]
- Djuris, J.; Djuric, Z. Modeling in the quality by design environment: Regulatory requirements and recommendations for design space and control strategy appointment. Int. J. Pharm. 2017, 533, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Yeom, S.B.; Ha, E.-S.; Kim, M.-S.; Jeong, S.H.; Hwang, S.-J.; Choi, D.H. Application of the discrete element method for manufacturing process simulation in the pharmaceutical industry. Pharmaceutics 2019, 11, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.S.; Choi, D.H. Application of mechanism-based modeling to predict drug quality during the pharmaceutical unit operations of granulation and compression: A review. J. Pharm. Investig. 2020, 50, 445–467. [Google Scholar] [CrossRef]
- Lawrence, X.Y.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar]
- Rogers, A.; Ierapetritou, M.; Engineering, C. Challenges and opportunities in modeling pharmaceutical manufacturing processes. Comput. Chem. Eng. 2015, 81, 32–39. [Google Scholar] [CrossRef]
- Stauffer, F.; Vanhoorne, V.; Pilcer, G.; Chavez, P.-F.; Schubert, M.; Vervaet, C.; De Beer, T. Managing active pharmaceutical ingredient raw material variability during twin-screw blend feeding. Eur. J. Pharm. Biopharm. 2019, 135, 49–60. [Google Scholar] [CrossRef]
- Badawy, S.I.F.; Menning, M.M.; Gorko, M.A.; Gilbert, D.L. Effect of process parameters on compressibility of granulation manufactured in a high-shear mixer. Int. J. Pharm. 2000, 198, 51–61. [Google Scholar] [CrossRef]
- Cavinato, M.; Bresciani, M.; Machin, M.; Bellazzi, G.; Canu, P.; Santomaso, A. The development of a novel formulation map for the optimization of high shear wet granulation. Chem. Eng. J. 2010, 164, 350–358. [Google Scholar] [CrossRef]
- Rahmanian, N.; Ghadiri, M.; Jia, X.; Stepanek, F. Characterisation of granule structure and strength made in a high shear granulator. Powder Technol. 2009, 192, 184–194. [Google Scholar] [CrossRef]
- Freitag, F.; Kleinebudde, P. How do roll compaction/dry granulation affect the tableting behaviour of inorganic materials? Comparison of four magnesium carbonates. Eur. J. Pharm. Sci. 2003, 19, 281–289. [Google Scholar] [CrossRef]
- Heiman, J.; Tajarobi, F.; Gururajan, B.; Juppo, A.; Abrahmsén-Alami, S. Roller compaction of hydrophilic extended release tablets—combined effects of processing variables and drug/matrix former particle size. AAPS PharmSciTech 2015, 16, 267–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, T.; Vom Bey, H.; Hanish, M.; Brockbank, K.; Armstrong, B. The influence of roller compaction processing variables on the rheological properties of granules. Asian J. Pharm. Sci. 2016, 11, 516–527. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Kaul, G.; Cai, C.; Chatlapalli, R.; Hernandez-Abad, P.; Ghosh, K.; Nagi, A. Quality by design case study: An integrated multivariate approach to drug product and process development. Int. J. Pharm. 2009, 382, 23–32. [Google Scholar] [CrossRef]
- Haware, R.V.; Tho, I.; Bauer-Brandl, A. Application of multivariate methods to compression behavior evaluation of directly compressible materials. Eur. J. Pharm. Biopharm. 2009, 72, 148–155. [Google Scholar] [CrossRef]
- Saharan, V.A. Current Advances in Drug Delivery through Fast Dissolving/Disintegrating Dosage Forms; Bentham Science Publishers: Sharjah, United Arab Emirates, 2017. [Google Scholar]
- Greco, K.; Bergman, T.L.; Bogner, R. Design and characterization of a laminar flow-through dissolution apparatus: Comparison of hydrodynamic conditions to those of common dissolution techniques. Pharm. Dev. Technol. 2011, 16, 75–87. [Google Scholar] [CrossRef]
- Choi, D.H.; Kim, K.H.; Park, J.S.; Jeong, S.H.; Park, K. Evaluation of drug delivery profiles in geometric three-layered tablets with various mechanical properties, in vitro–in vivo drug release, and Raman imaging. J. Control. Release 2013, 172, 763–772. [Google Scholar] [CrossRef]
- Issa, M.G.; Ferraz, H.G. Intrinsic dissolution as a tool for evaluating drug solubility in accordance with the biopharmaceutics classification system. Dissolution Technol. 2011, 18, 6–13. [Google Scholar] [CrossRef]
- Choi, D.H.; Kim, N.A.; Chu, K.R.; Jung, Y.J.; Yoon, J.-H.; Jeong, S.H. Material properties and compressibility using Heckel and Kawakita equation with commonly used pharmaceutical excipients. J. Pharm. Investig. 2010, 40, 237–244. [Google Scholar]
- Han, J.K.; Shin, B.S.; Choi, D.H. Comprehensive study of intermediate and critical quality attributes for process control of high-shear wet granulation using multivariate analysis and the quality by design approach. Pharmaceutics 2019, 11, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.H.; Lim, J.Y.; Shin, S.; Choi, W.J.; Jeong, S.H.; Lee, S. A novel experimental design method to optimize hydrophilic matrix formulations with drug release profiles and mechanical properties. J. Pharm. Sci. 2014, 103, 3083–3094. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Choi, D.H.; Kim, M.S.; Jeong, S.H. Effects of granulation process variables on the physical properties of dosage forms by combination of experimental design and principal component analysis. Asian J. Pharm. Sci. 2019, 14, 287–304. [Google Scholar] [CrossRef]
- Letellier, P.; Mayaffre, A.; Turmine, M. Drop size effect on contact angle explained by nonextensive thermodynamics. Young’s equation revisited. J. Colloid Interface Sci. 2007, 314, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H.; Williams, L.J. Principal component analysis. In Wiley Interdisciplinary Reviews: Computational Statistics; Wiley: Hoboken, NJ, USA, 2010; pp. 433–459. [Google Scholar]
- Wold, S.; Esbense, K.; Geladi, P. Principal companent analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Ganti, A. Correlation Coefficient. Corp. Financ. Account. 2020, 9, 145–152. [Google Scholar]
- Kassa, W.-E.; Billabert, A.-L.; Faci, S.; Algani, C. Electrical modeling of semiconductor laser diode for heterodyne RoF system simulation. IEEE J. Quantum Electron. 2013, 49, 894–900. [Google Scholar] [CrossRef]
- Wang, C.; Tang, C.-H.; Fu, X.; Huang, Q.; Zhang, B. Granular size of potato starch affects structural properties, octenylsuccinic anhydride modification and flowability. Food Chem. 2016, 212, 453–459. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Sui, Y.; She, Z.; Zhai, W.; Wang, C.; Deng, Y. Effect of particle size on solubility, dissolution rate, and oral bioavailability: Evaluation using coenzyme Q10 as naked nanocrystals. Int. J. Nanomed. 2012, 7, 5733. [Google Scholar]
- Shipar, M.A.H.; Wadhwa, A.; Varughese, C. Affect of granule sizes, types and concentrations of lubricants and compression forces on tablet properties. Int. J. Pharm. Sci. Res. 2014, 5, 4893. [Google Scholar]
- Van den Ban, S.; Goodwin, D.J. The impact of granule density on tabletting and pharmaceutical product performance. Pharm. Res. 2017, 34, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Jain, S. Quality by design (QBD): A comprehensive understanding of implementation and challenges in pharmaceuticals development. Int. J. Pharm. Sci. 2014, 6, 29–35. [Google Scholar]
- Narendar, D.; Arjun, N.; Someshwar, K.; Rao, Y.M. Quality by design approach for development and optimization of Quetiapine Fumarate effervescent floating matrix tablets for improved oral bioavailability. J. Pharm. Investig. 2016, 46, 253–263. [Google Scholar] [CrossRef]
- Carbinatto, F.M.; de Castro, A.D.; Evangelista, R.C.; Cury, B.S. Insights into the swelling process and drug release mechanisms from cross-linked pectin/high amylose starch matrices. Asian J. Pharm. Sci. 2014, 9, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Tang, N.; Lian, R.; Qi, J.; Wu, W. Understanding the relationship between wettability and dissolution of solid dispersion. Int. J. Pharm. 2014, 465, 25–31. [Google Scholar] [CrossRef]
- Dhenge, R.M.; Fyles, R.S.; Cartwright, J.J.; Doughty, D.G.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Granule properties. Chem. Eng. J. 2010, 164, 322–329. [Google Scholar] [CrossRef]
- Davies, W.L.; Gloor, W.T., Jr. Batch production of pharmaceutical granulations in a fluidized bed I: Effects of process variables on physical properties of final granulation. J. Pharm. Sci. 1971, 60, 1869–1874. [Google Scholar] [CrossRef]
- Alkan, M.; Yuksel, A. Granulation in a fluidized bed II Effect of binder amount on the final granules. Drug Dev. Ind. Pharm. 1986, 12, 1529–1543. [Google Scholar] [CrossRef]
- Mirza, Z.; Liu, J.; Glocheux, Y.; Albadarin, A.B.; Walker, G.M.; Mangwandi, C. Effect of impeller design on homogeneity, size and strength of pharmaceutical granules produced by high-shear wet granulation. Particuology 2015, 23, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Rahmanian, N.; Ghadiri, M.; Ding, Y. Effect of scale of operation on granule strength in high shear granulators. Chem. Eng. Sci. 2008, 63, 915–923. [Google Scholar] [CrossRef]
- Shi, L.; Feng, Y.; Sun, C.C. Massing in high shear wet granulation can simultaneously improve powder flow and deteriorate powder compaction: A double-edged sword. Eur. J. Pharm. Sci. 2011, 43, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.I.; Narang, A.S.; LaMarche, K.; Subramanian, G.; Varia, S.A. Mechanistic basis for the effects of process parameters on quality attributes in high shear wet granulation. Int. J. Pharm. 2012, 439, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Allesø, M.; Holm, R.; Holm, P. Roller compaction scale-up using roll width as scale factor and laser-based determined ribbon porosity as critical material attribute. Eur. J. Pharm. Sci. 2016, 87, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Al-Rabadi, G.J.; Gilbert, R.G.; Gidley, M.J. Effect of particle size on kinetics of starch digestion in milled barley and sorghum grains by porcine alpha-amylase. J. Cereal Sci. 2009, 50, 198–204. [Google Scholar] [CrossRef]
- Schafer, T. Comparison between granule growth in a horizontal and a vertical high speed mixer: I. Granulation of dicalcium phosphate. Arch. Pharm. Chem. Sci. 1986, 14, 209–224. [Google Scholar]
- Nguyen, V.; Nguyen, Q.; Zhang, Y.; Lim, C.; Khoo, B. Effect of particle size on erosion characteristics. Wear 2016, 348, 126–137. [Google Scholar] [CrossRef]
- Goodwin, J.; Sage, W.; Tilly, G. Study of erosion by solid particles. Proc. Inst. Mech. 1969, 184, 279–292. [Google Scholar] [CrossRef]
- Zerbe, H.G.; Krumme, M. Smartrix system: Design characteristics and release properties of a novel erosion-controlled oral delivery system. In Modified-Release Drug Delivery Technology; Marcel Dekker: New York, NY, USA, 2002; pp. 59–76. [Google Scholar]
- Woyna-Orlewicz, K.; Jachowicz, R. Analysis of wet granulation process with Plackett–Burman design–case study. Acta Pol. Pharm. 2011, 68, 725–733. [Google Scholar]
- Pandey, P.; Tao, J.; Chaudhury, A.; Ramachandran, R.; Gao, J.Z.; Bindra, D.S. A combined experimental and modeling approach to study the effects of high-shear wet granulation process parameters on granule characteristics. Pharm. Dev. Technol. 2013, 18, 210–224. [Google Scholar] [CrossRef]
- Souihi, N.; Josefson, M.; Tajarobi, P.; Gururajan, B.; Trygg, J. Design space estimation of the roller compaction process. Ind. Eng. Chem. Res. 2013, 52, 12408–12419. [Google Scholar] [CrossRef]
- Meda, U.S.; Rakesh, S.S.; Chandra, L.B. Impact of particle size on content uniformity. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 9369–9374. [Google Scholar]








| Quality Attributes | Target | Justification | Critical | |
|---|---|---|---|---|
| Dosage form | Bilayer tablet with coating film | Pharmaceutical equivalence requirement: same dosage form. | Not critical | |
| Dosage design | Bilayer tablet composed with immediate release and sustained release containing 10 mg of dapagliflozin and 1000 mg of metformin, respectively | Sustained release design needed to meet label claims. | Not critical | |
| Route of administration | Oral | Pharmaceutical equivalence requirement: same route of administration. | Not critical | |
| Dosage strength | dapagliflozin 10 mg/metformin 1000 mg | Pharmaceutical equivalence requirement: same strength. | Not critical | |
| Pharmacokinetics | Cmax within 2 h of dapagliflozin consumption under the fasting state and Cmax withn 4.0 to 8.0 h of metformin consumption; bioequivalent to XIGDUO™ XR | Bioequivalence requirement. | Not critical | |
| Stability | At accelerated conditions: 40 °C/75% RH At long term storage condition: 25 °C/60% RH | Equivalent to or better than the shelf-life of XIGDUO™ XR. | Not critical | |
| Drug product quality attributes | Assay | 90% to 110% w/w of label claim | Assay variability will affect safety and efficacy. Both material attributes and process parameters may affect the assay of the drug product. Thus, the assay should be evaluated throughout the product and process development. | Critical |
| Content uniformity (C.U.) | Conforms to USP<905> uniformity of dosage units | Variability in content uniformity will affect safety and efficacy. Both formulation and process variables impact content uniformity, so this CQA should be evaluated throughout product and process development. | Critical | |
| Swelling property | Similar to XIGDUO™ XR | The swelling property, gel strength, weight gain, and mass loss of tablets may affect safety and efficacy as these are directly correlated with dissolution that will affect bioavailability. | Critical | |
| Weight gain | ||||
| Mass loss | ||||
| Gel strength | ||||
| Dissolution | Metformin: 10% to 40% after 1 h; 40% to 70% after 3 h; more than 75% after 10 h. Dapagliflozin: more than 70% after 30 min | Failure to meet the dissolution specifications can affect bioavailability. Process variables affect the dissolution profile. | Critical | |
| Hardness | Ranging from 17.0 kp to 18.0 kp for metformin and from 27.0 kp to 28.0 kp for dapagliflozin | An extremely hard tablet could indicate excessive bonding potential between API and the excipients, which can prevent the proper dissolution of the tablet needed for accurate dosing. | Critical | |
| Friability | Not more than 1.0% w/w | Friability is a routine test as per the compendial requirements of tablets. A target of less than 1.0% w/w of mean weight loss assures a low impact on patient safety and efficacy and minimizes customer complaints. | Critical | |
| Contact angle | Similar to XIGDUO™ XR | The contact angle of tablets may affect safety and efficacy as it is directly correlated with dissolution that will affect bioavailability. Thus, it affects bioavailability. Thus, the contact angle should be evaluated throughout product and process development. | Critical | |
| Identification | Pharmaceutical equivalence requirement: Must meet the same applicable (quality) standards (i.e., identity, assay, purity, and quality). | Not critical | ||
| Degradation products | Not critical | |||
| Residual solvents | Not critical | |||
| Microbial limits | Not critical | |||
| Physical attributes | Not critical | |||
| Intermediate product quality attributes | Intrinsic dissolution rate | Similar to XIGDUO™ XR | Intrinsic dissolution rate and solubility are the main physicochemical aspects pertaining to drug absorption. The intrinsic dissolution test may offer greater correlation to the in vivo dissolution dynamic than the solubility test. Thus, the intrinsic dissolution test should be investigated throughout product and process development. | Critical |
| Granule size | The particle size affects flowability and content uniformity. Thus, size should be evaluated throughout product development. | Critical | ||
| Granule flowability | Granule flowability is relative to granule size, assay, CU, and dissolution. Thus, granule flowability should be evaluated throughout the product and process development. | Critical | ||
| Container closure system | Container closure system qualified as suitable for this drug product | Needed to achieve the target shelf-life and to ensure tablet integrity during shipping. | Not critical | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Chun, M.H.; Choi, D.H. Control Strategy for Process Development of High-Shear Wet Granulation and Roller Compaction to Prepare a Combination Drug Using Integrated Quality by Design. Pharmaceutics 2021, 13, 80. https://doi.org/10.3390/pharmaceutics13010080
Kim JY, Chun MH, Choi DH. Control Strategy for Process Development of High-Shear Wet Granulation and Roller Compaction to Prepare a Combination Drug Using Integrated Quality by Design. Pharmaceutics. 2021; 13(1):80. https://doi.org/10.3390/pharmaceutics13010080
Chicago/Turabian StyleKim, Ji Yeon, Myung Hee Chun, and Du Hyung Choi. 2021. "Control Strategy for Process Development of High-Shear Wet Granulation and Roller Compaction to Prepare a Combination Drug Using Integrated Quality by Design" Pharmaceutics 13, no. 1: 80. https://doi.org/10.3390/pharmaceutics13010080