Preparation, Characterization, and In Vivo Pharmacokinetic Evaluation of Polyvinyl Alcohol and Polyvinyl Pyrrolidone Blended Hydrogels for Transdermal Delivery of Donepezil HCl
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydrogels
2.3. Determination of Gel Fraction (%)
2.4. Determination of Swelling Ratio (%)
2.5. Weight Loss (%)
2.6. Determination of the Mechanical Properties
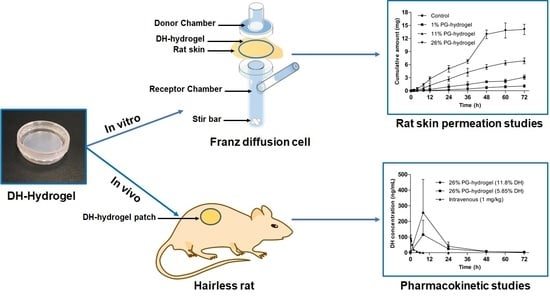
2.7. In Vitro Skin Permeation Study
2.8. Animals
2.9. Pharmacokinetic Study in Hairless Rats
2.10. Analysis of Plasma Donepezil Hydrochloride (DH) Levels
2.11. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Hydrogels
3.1.1. Determination of the Gel Fraction (%)
3.1.2. Determination of the Swelling Ratio (%)
3.1.3. Determination of Weight Loss (%)
3.1.4. Mechanical Properties Determinations
3.2. In Vitro Skin Permeation Study
3.3. Pharmacokinetic Profiles of the DH Hydrogel-Patch in Hairless Rats
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choi, J.; Choi, M.-K.; Chong, S.; Chung, S.-J.; Shim, C.-K.; Kim, D.-D. Effect of fatty acids on the transdermal delivery of donepezil: In vitro and in vivo evaluation. Int. J. Pharm. 2012, 422, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Disease International. World Alzheimer Report 2015. Available online: https://www.alz.co.uk/research/statistics (accessed on 29 January 2020).
- Association, A.S. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Bartus, R.T.; Dean, R.r.; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef]
- Tariot, P.; Salloway, S.; Yardley, J.; Mackell, J.; Moline, M. Long-term safety and tolerability of donepezil 23 mg in patients with moderate to severe Alzheimer’s disease. BMC Res. Notes 2012, 5, 283. [Google Scholar] [CrossRef] [Green Version]
- Subedi, R.K.; Ryoo, J.-P.; Moon, C.; Chun, M.-K.; Choi, H.-K. Formulation and in vitro evaluation of transdermal drug delivery system for donepezil. J. Pharm. Investig. 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Han, M.-R.; Kim, Y.-H.; Shin, S.-W.; Nam, S.-Y.; Park, J.-H. Tip-loaded dissolving microneedles for transdermal delivery of donepezil hydrochloride for treatment of Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2016, 105, 148–155. [Google Scholar] [CrossRef]
- Saluja, S.; Kasha, P.C.; Paturi, J.; Anderson, C.; Morris, R.; Banga, A.K. A novel electronic skin patch for delivery and pharmacokinetic evaluation of donepezil following transdermal iontophoresis. Int. J. Pharm. 2013, 453, 395–399. [Google Scholar] [CrossRef]
- Galipoğlu, M.; Erdal, M.S.; Güngör, S. Biopolymer-based transdermal films of donepezil as an alternative delivery approach in Alzheimer’s disease treatment. AAPS PharmSciTech 2015, 16, 284–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sozio, P.; Cerasa, L.S.; Marinelli, L.; Di Stefano, A. Transdermal donepezil on the treatment of Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2012, 8, 361. [Google Scholar]
- Hajjar, B.; Zier, K.-I.; Khalid, N.; Azarmi, S.; Löbenberg, R. Evaluation of a microemulsion-based gel formulation for topical drug delivery of diclofenac sodium. J. Pharm. Investig. 2018, 48, 351–362. [Google Scholar] [CrossRef]
- Rastogi, V.; Yadav, P.; Verma, N.; Verma, A. Preparation and characterization of transdermal mediated microemulsion delivery of T4 bacteriophages against E. coli bacteria: A novel anti-microbial approach. J. Pharm. Investig. 2018, 48, 393–407. [Google Scholar] [CrossRef]
- Bashyal, S.; Lee, S. Delivery of biopharmaceuticals using combination of liposome and iontophoresis: A review. J. Pharm. Investig. 2015, 45, 611–624. [Google Scholar] [CrossRef]
- Lee, A.-R.C. Microneedle-mediated delivery of cosmeceutically relevant nucleoside and peptides in human skin: Challenges and strategies for dermal delivery. J. Pharm. Investig. 2019, 49, 587–601. [Google Scholar]
- Karande, P.; Mitragotri, S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim. Biophys. Acta 2009, 1788, 2362–2373. [Google Scholar] [CrossRef] [Green Version]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009; pp. 592–593. [Google Scholar]
- Trommer, H.; Neubert, R. Overcoming the stratum corneum: The modulation of skin penetration. Skin Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef]
- Dai, Y.N.; Li, P.; Zhang, J.P.; Wang, A.Q.; Wei, Q. A novel pH sensitive N-succinyl chitosan/alginate hydrogel bead for nifedipine delivery. Biopharm. Drug Dispos. 2008, 29, 173–184. [Google Scholar] [CrossRef]
- Wona, G.; Janik, H. Review: Synthetic polymer hydrogels forbiomedical application. Chem. Chem. Technol. 2010, 4, 297–304. [Google Scholar]
- Rac, V.; Lević, S.; Balanč, B.; Graells, B.O.; Bijelić, G. PVA Cryogel as model hydrogel for iontophoretic transdermal drug delivery investigations. Comparison with PAA/PVA and PAA/PVP interpenetrating networks. Colloids Surf. B Biointerfaces 2019, 180, 441–448. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Mansour, L.M.; Taj, B.M.; Mokhtari, M. Synthesis and swelling characterization of cross-linked PVP/PVA hydrogels. Iran. Polym. J. 2005, 14, 1022–1030. [Google Scholar]
- Nho, Y.-C.; Lim, Y.-M.; Gwon, H.-J.; Choi, E.-K. Preparation and characterization of PVA/PVP/glycerin/antibacterial agent hydrogels using γ-irradiation followed by freeze-thawing. Korean J. Chem. Eng. 2009, 26, 1675–1678. [Google Scholar] [CrossRef]
- Thomas, J.; Lowman, A.; Marcolongo, M. Novel associated hydrogels for nucleus pulposus replacement. J. Biomed. Mater. Res. A 2003, 67, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Harthi, S.A.; Alavi, S.E.; Radwan, M.A.; El Khatib, M.M.; AlSarra, I.A. Nasal delivery of donepezil HCl-loaded hydrogels for the treatment of Alzheimer’s disease. Sci. Rep. 2019, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Razzak, M.T.; Darwis, D. Irradiation of polyvinyl alcohol and polyvinyl pyrrolidone blended hydrogel for wound dressing. Radiat. Phys. Chem. 2001, 62, 107–113. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly (vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. In Biopolymers PVA Hydrogels, Anionic Polymerisation Nanocomposites; Springer: New York, NY, USA, 2000; pp. 37–65. [Google Scholar]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef]
- Soazo, M.; Báez, G.; Barboza, A.; Busti, P.A.; Rubiolo, A.; Verdini, R.; Delorenzi, N. Heat treatment of calcium alginate films obtained by ultrasonic atomizing: Physicochemical characterization. Food Hydrocoll. 2015, 51, 193–199. [Google Scholar] [CrossRef]
- Travan, A.; Scognamiglio, F.; Borgogna, M.; Marsich, E.; Donati, I.; Tarusha, L.; Grassi, M.; Paoletti, S. Hyaluronan delivery by polymer demixing in polysaccharide-based hydrogels and membranes for biomedical applications. Carbohydr. Polym. 2016, 150, 408–418. [Google Scholar] [CrossRef] [Green Version]
- Noh, G.; Keum, T.; Seo, J.-E.; Bashyal, S.; Eum, N.-S.; Kweon, M.; Lee, S.; Sohn, D.; Lee, S. Iontophoretic transdermal delivery of Human Growth Hormone (hGH) and the combination effect of a new type Microneedle, Tappy Tok Tok®. Pharmaceutics 2018, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.S.; Seo, J.E.; Kim, M.S.; Kang, M.H.; Oh, D.H.; Jeon, S.O.; Jeong, S.H.; Choi, Y.W.; Lee, S. A retinyl palmitate-loaded solid lipid nanoparticle system: Effect of surface modification with dicetyl phosphate on skin permeation in vitro and anti-wrinkle effect in vivo. Int. J. Pharm. 2013, 452, 311–320. [Google Scholar] [CrossRef]
- Pappa, H.; Farrú, R.; Vilanova, P.O.; Palacios, M.; Pizzorno, M.a.T. A new HPLC method to determine Donepezil hydrochloride in tablets. J. Pharm. Biomed. Anal. 2002, 27, 177–182. [Google Scholar] [CrossRef]
- Bashyal, S.; Seo, J.-E.; Keum, T.; Noh, G.; Choi, Y.W.; Lee, S. Facilitated permeation of insulin across TR146 cells by cholic acid derivatives-modified elastic bilosomes. Int. J. Nanomed. 2018, 13, 5173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geerts, H.; Guillaumat, P.-O.; Grantham, C.; Bode, W.; Anciaux, K.; Sachak, S. Brain levels and acetylcholinesterase inhibition with galantamine and donepezil in rats, mice, and rabbits. Brain Res. 2005, 1033, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Bhateria, M.; Ramakrishna, R.; Pakala, D.B.; Bhatta, R.S. Development of an LC–MS/MS method for simultaneous determination of memantine and donepezil in rat plasma and its application to pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1001, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Webster, T.J.; Sinha, A. Evolution of PVA gels prepared without crosslinking agents as a cell adhesive surface. J. Mater. Sci. Mater. Med. 2011, 22, 1763–1772. [Google Scholar] [CrossRef]
- Kim, J.O.; Park, J.K.; Kim, J.H.; Jin, S.G.; Yong, C.S.; Li, D.X.; Choi, J.Y.; Woo, J.S.; Yoo, B.K.; Lyoo, W.S. Development of polyvinyl alcohol–sodium alginate gel-matrix-based wound dressing system containing nitrofurazone. Int. J. Pharm. 2008, 359, 79–86. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Tamer, T.M.; El-Meligy, M.A.; Eldin, M.S.M. Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arab. J. Chem. 2015, 8, 38–47. [Google Scholar] [CrossRef]
- Mi, F.L.; Shyu, S.S.; Lee, S.T.; Wong, T.B. Kinetic study of chitosan-tripolyphosphate complex reaction and acid-resistive properties of the chitosan-tripolyphosphate gel beads prepared by in-liquid curing method. J. Polym. Sci. Pol. Phys. 1999, 37, 1551–1564. [Google Scholar] [CrossRef]
- Kickhöfen, B.; Wokalek, H.; Scheel, D.; Ruh, H. Chemical and physical properties of a hydrogel wound dressing. Biomaterials 1986, 7, 67–72. [Google Scholar] [CrossRef]
- Hwang, M.-R.; Kim, J.O.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S.; Han, S.S. Gentamicin-loaded wound dressing with polyvinyl alcohol/dextran hydrogel: Gel characterization and in vivo healing evaluation. AAPS PharmsciTech 2010, 11, 1092–1103. [Google Scholar] [CrossRef] [Green Version]
- Corium International Inc. Lead Investigational Product. Available online: http://www.coriumintl.com (accessed on 9 March 2020).
- Sinha, V.; Kaur, M.P. Permeation enhancers for transdermal drug delivery. Drug Dev. Ind. Pharm. 2000, 26, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Yamane, M.; Williams, A.; Barry, B. Terpene penetration enhancers in propylene glycol/water co-solvent systems: Effectiveness and mechanism of action. J. Pharm. Pharmacol. 1995, 47, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeong, N.Y.; Oh, S.Y. Modulation of electroosmosis and flux through skin: Effect of propylene glycol. Arch. Pharm. Res. 2014, 37, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.; De Vries, M.; Gooris, G.; Bras, W.; Brussee, J.; Ponec, M. Thermodynamic and structural aspects of the skin barrier. J. Control Release 1991, 15, 209–219. [Google Scholar] [CrossRef]
- Haque, T.; Talukder, M.M.U. Chemical enhancer: A simplistic way to modulate barrier function of the stratum corneum. Adv. Pharm. Bull. 2018, 8, 169. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, D.; Hirata, K.; Hadgraft, J.; Lane, M.E. Influence of skin penetration enhancers on skin barrier function and skin protease activity. Eur. J. Pharm. Sci. 2014, 51, 118–122. [Google Scholar] [CrossRef]
- Trottet, L.; Merly, C.; Mirza, M.; Hadgraft, J.; Davis, A. Effect of finite doses of propylene glycol on enhancement of in vitro percutaneous permeation of loperamide hydrochloride. Int. J. Pharm. 2004, 274, 213–219. [Google Scholar] [CrossRef]
- Mendes, M.; Nunes, S.; Sousa, J.; Pais, A.; Vitorino, C. Expanding transdermal delivery with lipid nanoparticles: A new drug-in-NLC-in-adhesive design. Mol. Pharm. 2017, 14, 2099–2115. [Google Scholar] [CrossRef]







| Molecular Structure |  |
|---|---|
| Molecular weight | 415.96 g/mol |
| H-bond donor | 1 |
| H-bond acceptor | 4 |
| Log P | 4.27 |
| pKa | 8.9 |
| Ingredients | 0% PG-Hydrogel | 1% PG-Hydrogel | 11% PG-Hydrogel | 26% PG-Hydrogel |
|---|---|---|---|---|
| PVA | 7.5 | 7.5 | 7.5 | 7.5 |
| PVP K-90 | 8 | 8 | 8 | 8 |
| PVP K-30 | 2 | 2 | 2 | 2 |
| PG | 0 | 1 | 11 | 26 |
| Glycerol | 1 | 1 | 1 | 1 |
| DH | 11.7 | 11.7 | 11.7 | 11.7 |
| Water | 69.8 | 68.8 | 58.8 | 43.8 |
| Total | 100 | 100 | 100 | 100 |
| Observation (Organoleptic Property) |
|---|
|
|
|
|
|
|
|
|
|
|
| Hydrogel | Js (mg·cm−2·h−1) | Kp (cm/h) × 10−4 | Lag Time (h) | Enhancement Ratio (ER) |
|---|---|---|---|---|
| Control | 0.009 ± 0.002 | 2.32 ± 0.65 | 3.76 ± 0.66 | 1.00 |
| 1% PG-hydrogel | 0.024 ± 0.004 * | 6.60 ± 1.08 * | 2.10 ± 3.05 | 2.85 |
| 11% PG-hydrogel | 0.054 ± 0.005 ***,## | 14.62 ± 1.40 ***,## | 0.23 ± 2.67 | 6.30 |
| 26% PG-hydrogel | 0.110 ± 0.009 ***,###,$$$ | 29.95 ± 2.49 ***,###,$$$ | 0.63 ± 1.04 | 12.90 |
| Parameter | IV | DH Hydrogel-Patch | |
|---|---|---|---|
| Dose | 1 mg/kg | 5.85% | 11.7% |
| Tmax (h) | - | 8.0 ± 0.0 | 8.0 ± 0.0 |
| Cmax (ng/mL) | 93.4 ± 18.9 | 115.2 ± 41.3 | 255.4 ± 95.3 |
| AUC0→last (ng·h/mL) | 133.4 ± 23.5 | 1815.1 ± 631.9 | 3420.3 ± 1087.6 |
| F | - | 0.35 | 0.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashyal, S.; Shin, C.Y.; Hyun, S.M.; Jang, S.W.; Lee, S. Preparation, Characterization, and In Vivo Pharmacokinetic Evaluation of Polyvinyl Alcohol and Polyvinyl Pyrrolidone Blended Hydrogels for Transdermal Delivery of Donepezil HCl. Pharmaceutics 2020, 12, 270. https://doi.org/10.3390/pharmaceutics12030270
Bashyal S, Shin CY, Hyun SM, Jang SW, Lee S. Preparation, Characterization, and In Vivo Pharmacokinetic Evaluation of Polyvinyl Alcohol and Polyvinyl Pyrrolidone Blended Hydrogels for Transdermal Delivery of Donepezil HCl. Pharmaceutics. 2020; 12(3):270. https://doi.org/10.3390/pharmaceutics12030270
Chicago/Turabian StyleBashyal, Santosh, Chang Yell Shin, Sang Min Hyun, Sun Woo Jang, and Sangkil Lee. 2020. "Preparation, Characterization, and In Vivo Pharmacokinetic Evaluation of Polyvinyl Alcohol and Polyvinyl Pyrrolidone Blended Hydrogels for Transdermal Delivery of Donepezil HCl" Pharmaceutics 12, no. 3: 270. https://doi.org/10.3390/pharmaceutics12030270