1. Introduction
Rumex acetosa L. (
R. acetosa), belonging to the Polygonaceae family, is a perennial herb that is listed in the Korean Food Code (Korea Food and Drug Administration) as a food material and has been used in folk remedies for gastrointestinal disorders and cutaneous diseases [
1]. Extracts of
R. acetosa have been reported to have various biological activities, including anti-ulcerogenic, anti-inflammatory, anti-proliferative, and anti-viral effects [
2,
3,
4]. They contain a number of bioactive compounds, including anthraquinones, flavonoids, and polysaccharides [
5]. In particular,
Rumex species contain abundant anthraquinones, including emodin, chrysophanol, and physcion in all parts of the plant, in free and glycoside forms [
6]. A difference in anthraquinone physiological activity between these forms has been described [
7]. Previous studies reported a number of quantitative methods of anthraquinones in plasma [
8,
9,
10]. However, we found that most of these studies focused on determining aglycones (free anthraquinones). Methods to quantify anthraquinone glycosides in plasma are rarely available.
As interest in natural drugs has increased in the pharmaceutical industry, research is underway to develop potential applications of R. acetosa, which has already proven its efficacy. Therefore, a simple and sensitive analytical method to examine bioactive anthraquinones in biological samples is needed to evaluate the potential of new treatments.
The aim of this study is to establish a simple, rapid, and sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) method to simultaneously quantify emodin (E), emodin-8-O-β-d-glucoside (EG), chrysophanol (C), chrysophanol-8-O-β-d-glucoside (CG), physcion (P), and physcion-8-O-β-d-glucoside (PG) in rat plasma within one chromatographic run. The method was applied to determine pharmacokinetic parameters after oral administration of R. acetosa 70% ethanol extract in rat. The results of this study might be helpful in the development of a new type of medicine using R. acetosa.
2. Materials and Methods
2.1. Materials
The plant of
R. acetosa L. (Polygonaceae) was collected from the Sancheong province of Korea in April 2014 and identified by Mi-Jeong Ahn of the College of Pharmacy, Gyeongsang National University (Jinju, Korea). The voucher specimen (APG-1403) was deposited in the Herbarium of the College of Pharmacy, Gyeongsang National University. The standards of the six anthraquinones (E, EG, C, CG, P, and PG) were isolated from the whole part of
R. acetosa and their structures (
Figure 1) were elucidated using spectroscopy such as MS and nuclear magnetic resonance spectroscopy (data not shown) [
11]. The purity of anthraquinone compounds isolated from
R. acetosa was confirmed to be more than 95% by NMR and HPLC-UV. Diclofenac used as an internal standard (IS) was purchased from Sigma Aldrich (St. Louis, MO, USA). HPLC-grade acetonitrile, methanol, and water were products of Fisher Scientific Korea Ltd. (Seoul, Korea). All reagents were analytical grade.
2.2. Chromatographic Condition
The analysis was performed on an Agilent 1260 series (Agilent Technologies, Waldbronn, Germany) HPLC system. Chromatographic separation of the samples was carried out on a Kinetex Biphenyl column (100 × 3.0 mm, 2.6 μm, 110 Å, Phenomenex, Torrance, CA, USA). The mobile phase consisted of 2 mM ammonium formate (pH 6) in water (A) and 2 mM ammonium formate (pH 6) in methanol (B). The gradient program was used at a flow rate of 0.3 mL/min while maintaining the column temperature at 40 °C. The mobile phase initial composition of 25% B was maintained for 2 min. It was then increased linearly from 25% to 95% B for 0.5 min and held for 7 min. The gradient was then changed back to the initial condition for 0.5 min and kept at the initial condition for 3 min. The total analysis time was 13 min for each sample. The injection volume was 15 μL.
2.3. Mass Spectrometric Condition
The mass spectrometric detection was performed on an Agilent 6460 triple-quadruple mass spectrometer (Agilent Technologies, Singapore) with an electrospray ionization source. It was operated in the negative ion detection mode because of its higher sensitivity than that in the positive ionization mode on multiple reaction monitoring (MRM). The data were acquired and processed using Mass Hunter Workstation B.06.00 software (Agilent Technologies, Singapore). The mass spectrometric parameters of each compound are summarized in
Table 1 [
12]. The MS spectra of the six anthraquinones are shown in
Figure 2. The source parameters were also optimized as follows: a drying gas flow and temperature at 6 L/min and 350 °C were used, respectively; the sheath gas flow and temperature were maintained at 12 L/min and 350 °C, respectively; the nebulizing gas (N
2) pressure was set at 25 psi; and the capillary and nozzle voltages were set at 3500 V and 500 V, respectively.
2.4. Preparation of R. acetosa Extract
The dried plant material (100 g) was ground and extracted with 70% ethanol. The extract was filtered using filter papers (Whatman No. 40) and concentrated through a rotary evaporator. The concentrate was lyophilized and stored at −80 °C. The exact amount was weighed and used as the samples for the animal studies. The contents of E, EG, C, CG, P, and PG in R. acetosa extract were 0.94 ± 0.15%, 1.29 ± 0.06%, 0.68 ± 0.09%, 0.77 ± 0.12%, 0.17 ± 0.02%, and 0.41 ± 0.05% (w/w), respectively. The values were expressed as mean ± standard deviation.
2.5. Preparation of the Calibration Standard and Quality Control (QC) Samples
The primary stock solutions of E, EG, C, CG, P, PG, and IS were prepared in dimethyl sulfoxide at a concentration of 1 mg/mL and stored at −80 °C. The mixture stock solutions to obtain the standard solutions were serially diluted in methanol. The IS stock solution of 5 ng/mL was prepared in methanol. The calibration standards were prepared by spiking 10 μL of above standard solutions into 90 μL of blank rat plasma to yield concentration ranges of 1–300 ng/mL for E, 20–300 ng/mL for P and C, 1–150 ng/mL for EG, 10–150 ng/mL for CG and PG. Twenty microliters of aliquots were prepared and stored at −80 °C until analysis.
The QC samples were prepared in the same way as the calibration samples for E, EG, C, CG, P, and PG in rat plasma at low, middle, and high concentrations. All the solutions were kept at −80 °C.
2.6. Sample Preparation
To 20 μL aliquot of the rat plasma samples, 60 μL of 5 ng/mL IS in methanol was added. The mixture was vortexed for 30 s and kept at 4 °C for 30 min. The mixture was centrifuged at 10,000× g for 10 min. The supernatant was transferred to an HPLC vial, and 15 μL of the processed sample was injected onto the LC-MS/MS system.
2.7. Method Validation
The method validation was performed according to the United States Food and Drug Administration’s guidance on bioanalytical method validation [
13].
2.7.1. Selectivity
The selectivity study was performed by comparing the chromatograms of the six different rat plasma samples to investigate the interference near the retention time of the analytes and the IS.
2.7.2. Calibration Curves and Sensitivity
The linearity of each calibration curve was determined by plotting the peak area ratio of the analyte to IS versus the plasma concentrations. The least-square method was used to achieve a linear regression equation. Sensitivity was defined by calculating the lower limit of detection and the lower limit of quantification (LLOQ) based on a signal-to-noise ratio of greater than 3 and 10, respectively. Besides signal-to-noise ratio, LLOQ values with acceptable precision and accuracy values were chosen. The criteria of precision and accuracy at LLOQ are within 20% relative standard deviation (RSD) for precision and between 80–120% for accuracy.
2.7.3. Precision and Accuracy
Precision and accuracy were investigated by analyzing six replicates of four QC levels on the same batch (intra-batch) and five different batches (inter-batch) of four QC levels (LLOQ, low QC, middle QC, and high QC). The intra- and inter-batch precision was expressed by RSD (%), and accuracy was evaluated by expressing it as a percentage of the theoretical value (the mean calculated concentration/nominal concentration) × 100%. The acceptance criteria are within 15% RSD except 20% at LLOQ for precision and ±15% of nominal concentrations except ±20% at LLOQ for accuracy.
2.7.4. Extraction Recovery and Matrix Effect
The extraction recovery was evaluated by comparing the peak area of the extracted sample with that of the post-extracted sample at three replicates of three QC levels. The matrix effect of the analytes was investigated by comparing the peak area of the post-extracted sample with the peak area obtained by the corresponding standard solutions in pH 7.4 buffer at three QC levels. Matrix effects were determined using the equation below.
2.7.5. Stability
The stability of the analytes in rat plasma was evaluated by analyzing triplicates of three QC levels at room temperature for 4 h (short-term stability), −80 °C for one month (long-term stability), three freeze-thaw cycles from −80 °C to room temperature (freeze and thaw stability), and 4 °C for 24 h (processed sample stability). The stability of analytes in stock solution was also evaluated. The peak areas obtained from freshly prepared stock solutions were compared with stock solutions stored for 4 h at room temperature.
2.8. Pharmacokinetic Study
Male Sprague‒Dawley rats (8-week-old, weighing 250 ± 10 g) were obtained from Koatech (Pyeongtaek, Korea). They were housed and acclimated in the Animal Laboratory, Gyeongsang National University, under controlled temperature and humidity and regular 12 h light cycle, freely accessible to food and water for 7 days before the experiment. The rats were cannulated into the carotid artery and allowed to recover for one day. Before the pharmacokinetic study, all rats were fasted for 12 h with free access to water. R. acetosa extract suspended in a solution (ethanol:polysorbate 80:water = 1:2:7, v/v/v) was orally administered to the three rats at a dose of 2 g/kg. The calculated doses of compounds based on the contents in the extract were 18.8, 25.8, 13.6, 15.4, 3.4, and 8.2 mg/kg for E, EG, C, CG, P, and PG, respectively. Blood samples (100 µL) were collected via the cannulated carotid vessel at 0, 15, 30, 45 min, 1, 2, 3, 4, 6, 8, 12, and 24 h after oral administration. To collect plasma, the blood samples were immediately centrifuged at 10,000× g for 5 min. All plasma samples were stored at −80 °C until analysis. All experimental procedures of the animal study were approved (GNU-130618-R0038) by the Animal Care and Use Committee of Gyeongsang National University, Korea.
4. Discussion
The objective of this study was to develop a bioanalytical method that simultaneously quantified the bioactive glycosides and aglycones of anthraquinones. The developed LC-MS/MS method could quantify six anthraquinones simultaneously in rat plasma in an accurate, reproducible, and simple way. Reported bioanalytical methods which simultaneously determine both aglycones and glycosides of anthraquinones are rarely available. In this study, simultaneous determination achieved by using biphenyl column. The column could prolong retention time of hydrophilic glycosides compare to C18 column at the same mobile phase composition.
There was no interfering peak when the compound mixture was spiked to blank rat plasma. However, there were small peaks appeared near CG and PG peaks after oral administration of plant extract. It is suggested that those peaks came from the extract or the metabolites of components in the extract. It is known that emodin is extensively glucuronized after absorption [
14] and the molecular weight of emodin glucuronide is same as PG. There is some possibility that emodin glucuronide could interfere PG. However, the MS/MS fragment pattern of emodin glucuronide is different from PG. Emodin glucuronide might cause little interference. We could quantify CG and PG by adjusting the baselines because the interfering peaks were small.
A simple and rapid sample preparation was utilized on a low volume of rat plasma sample (20 µL) by using methanol as a precipitating agent with appropriate sensitivity compare to the reported methods [
8,
12,
15]. The comparison with reported analytical methods for aglycones and glycosides of anthraquinones was shown in
Table 6.
The method was acceptably validated and used to perform a pharmacokinetic study of anthraquinones after oral administration of
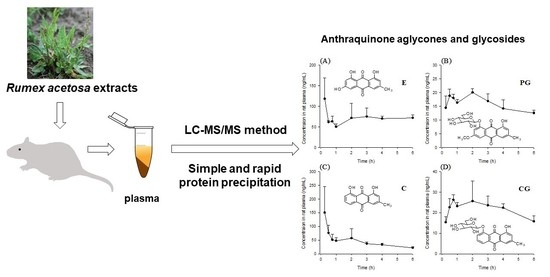
R. acetosa in rats. As shown in
Figure 4, C, E, CG, and PG could be detected in every rat from the first sampling time, 15 min. All of the studied anthraquinones were absorbed rapidly from rat gastrointestinal tract. Median T
max value of C and E was 15 min (
Table 5). This result was consistent with reported values [
8,
10]. Emodin could be detected for the longest time among four compounds even though the concentrations fluctuated. The fluctuated concentration was also reported in other pharmacokinetic studies of emodin in rats [
9,
15]. This was possibly due to enterohepatic circulation [
16]. In some other works [
17], emodin rapidly and extensively metabolized to form its glucuronide and the parent form was almost undetectable after administration of emodin even the doses were similar (40 mg/kg) to our study (18.8 mg/kg for E and 25.8 mg/kg for EG). Free emodin could be measured until 24 h after oral administration because of the low LLOQ level of emodin using the method developed in this work.
Generally, plant glycosides have been considered to be hydrolyzed to aglycones by microflora in the gastrointestinal tract before absorption [
18]. Glycosides have large molecular weights and low lipophilicity, so they might be difficult to be absorbed. However, recent studies show that emodin glycoside can be absorbed in an intact form after oral administration of plant extract [
8]. The absorption of the glycosides of anthraquinones in an intact form was confirmed by studying in vivo absorption in rats in this study. Pharmacokinetics of anthraquinone aglycones and their glycosides after oral administration of
R. acetosa was first evaluated. CG and PG were detected in rat plasma after oral administration of
R. acetosa extract. Interestingly, the T
max of C and that of CG were different after oral administration of the extract. It might be due to different lipophilicity. Glycosides and aglycones have been proposed to have different degrees of absorption and metabolic patterns. Note that second peaks in the plasma concentrations of aglycones were observed. This could be due to delayed absorption of aglycones hydrolyzed from glycosides by microflora in the gastrointestinal tract and enterohepatic circulation of anthraquinones [
16]. A number of published studies have reported pharmacokinetics of anthraquinones. However, we found that most of these studies focused on determining aglycones. Pharmacokinetics of anthraquinone glycosides is rarely available. Wang et al. [
12] recently reported the pharmacokinetics of anthraquinone aglycones and their glycosides in hyperlipidemic hamsters after administration of rhubarb. The plasma concentration-time profile patterns of anthraquinones were similar to our study even though the composition (dose ratio of compounds) of rhubarb extract might be quite different from
R. acetosa extract and physiological differences between rats and hamsters probably exist. T
max values of glycosides were slightly longer than aglycones. Similar to our results, emodin glucoside was not detected even though emodin could be detected until 36 h. It is suggested that emodin glycoside probably rapidly hydrolyzed to emodin and was poorly absorbed as an intact form in the gastrointestinal tract.
Our pharmacokinetic study has some limitations. The number of animals (n = 3) is not enough to achieve statistically significant pharmacokinetic parameters after administration of an herbal product. The pharmacokinetic parameters obtained in this study might b not sufficient to represent the animal population. Nevertheless, this study showed the possibility that our bioanalytical method could be used in pharmacokinetic studies of R. acetosa extract. Another issue to consider is that R. acetosa extract contained a number of other compounds besides anthraquinones. Further studies with a large sample size and studies of the effects of other compounds on the pharmacokinetics of anthraquinones are needed for better understanding of the pharmacokinetics of anthraquinones.