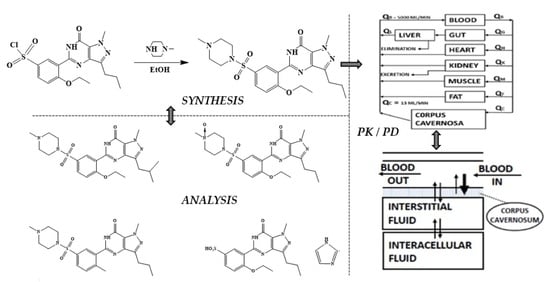
Sildenafil 4.0—Integrated Synthetic Chemistry, Formulation and Analytical Strategies Effecting Immense Therapeutic and Societal Impact in the Fourth Industrial Era
Abstract
:1. Introduction
2. Chemical Synthesis of Sildenafil
3. Physiochemical Properties
3.1. Solubility
3.2. Permeability
3.3. Thermogravimetric Analysis (TGA)
3.4. Differential Scanning Calorimetry (DSC)
3.5. Infrared Spectroscopy (IR)
3.6. X-ray Crystallography Data
3.7. NMR Spectroscopy
4. Pharmacology and Clinical Applications
4.1. Treatment of Erectile Dysfunction
4.2. Treatment against Pulmonary Arterial Hypertension
4.3. Sildenafil as a Potential Treatment Option for COVID-19
4.4. Potential Sildenafil Effectiveness on Cancer Treatment and Type 2 Diabetes
5. Pharmacokinetic and Pharmacodynamic Profile
5.1. Pharmacokinetic Profile of Sildenafil
5.2. Pharmacodynamic Properties

5.3. Drug Interactions
5.4. Pharmacokinetic/Pharmacodynamic Interactions
5.5. Pharmacokinetic Interactions with Endothelin Receptor Antagonists
5.6. Administration to Special Populations
5.6.1. Elderly
5.6.2. Patients with Renal and Hepatic Impairment
5.6.3. Patients with Pulmonary Arterial Hypertension (PAH)
5.6.4. Pediatric Populations
6. Alternative Administration Schemes
6.1. Oral Administration
6.1.1. Orally Disintegrating Tablets (ODT)
6.1.2. Oro-Dispersible Film (ODF)
6.1.3. Sublingual Delivery Systems
6.1.4. Oral Pediatric Suspensions
6.1.5. Chewable Tablets
6.1.6. Dry Foam Tablets
6.2. Intranasal Microemulsions
6.3. Inhalables of Controlled Release
6.4. Transdermal
6.5. Intravenous
7. Analytical Strategies for the Determination of Sildenafil in Pharmaceutical and Biological Matrices
7.1. Pharmaceutical Applications
7.1.1. Optical Methods
Spectrophotometric Methods
Other Spectroscopic Methods
7.1.2. Electroanalytical Methods
7.1.3. Chromatographic Methods
7.1.4. Specifications in an Assessment Report
API’s Specifications
Stability of API
Specifications of Tablet Formulations
Stability of Tablets Formulation
7.2. Bioanalytical Applications
7.2.1. LC-MS/MS
7.2.2. HPLC-UV
7.2.3. Voltammetry
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corbin, J.D.; Beasley, A.; Blount, M.A.; Francis, S.H. High lung PDE5: A strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem. Biophys. Res. Commun. 2005, 334, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, M.; Cardarelli, S.; Ragusa, F.; Saliola, M.; Biagioni, S.; Poiana, G.; Naro, F.; Massimi, M. Phosphodiesterase inhibitors: Could they be beneficial for the treatment of COVID-19? Int. J. Mol. Sci. 2020, 21, 5338. [Google Scholar] [CrossRef]
- Mario, L.; Roberto, M.; Marta, L.; Teresa, C.M.; Laura, M. Hypothesis of COVID-19 Therapy with Sildenafil. Int. J. Prev. Med. 2020, 11, 76. [Google Scholar]
- Mostafa, T. Could Oral Phosphodiesterase 5 Inhibitors Have a Potential Adjuvant Role in Combating COVID-19 Infection? Sex. Med. Rev. 2020, 9, 15–22. [Google Scholar] [CrossRef]
- Hyland, R.; Roe, E.G.H.; Jones, B.C.; Smith, D.A. Identification of the cytochrome P450 enzymes involved in the N-demethylation of sildenafil. Br. J. Clin. Pharmacol. 2001, 51, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warrington, J.S.; Shader, R.I.; von Moltke, L.L.; Greenblatt, D.J. In vitro biotransformation of sildenafil (Viagra): Identification of human cytochromes and potential drug interactions. Drug Metab. Dispos. 2000, 4, 392–397. [Google Scholar]
- Walker, D.K. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica 1999, 29, 297–310. [Google Scholar] [CrossRef]
- Terrett, N.K.; Bell, A.S.; Brown, D.; Ellis, P. Sildenafil (VIAGRATM), a potent and selective inhibitor of type 5 cGMP phosphodiesterase with utility for the treatment of male erectile dysfunction. Bioorganic Med. Chem. Lett. 1996, 6, 1819–1824. [Google Scholar] [CrossRef]
- Dunn, P.J.; Wood, A.S. Patent: Process for Preparation of Sildenafil by Cyclization. 1997. Available online: https://patents.google.com/patent/EP0812845B1/en (accessed on 12 April 2021).
- Dale, D.J.; Dunn, P.J.; Golightly, C.; Hughes, M.L.; Levett, P.C.; Pearce, A.K.; Searle, P.M.; Ward, G.; Wood, A.S. The chemical development of the commercial route to sildenafil: A case history. Org. Process Res. Dev. 2000, 4, 17–22. [Google Scholar] [CrossRef]
- Dunn, P.J.; Galvin, S.; Hettenbach, K. The development of an environmentally benign synthesis of sildenafil citrate (ViagraTM) and its assessment by Green Chemistry metrics. Green Chem. 2004, 6, 43–48. [Google Scholar] [CrossRef]
- Melnikov, P.; Corbi, P.P.; Cuin, A.; Cavicchioli, M.; Guimarães, W.R. Physicochemical properties of sildenafil citrate (Viagra) and sildenafil base. J. Pharm. Sci. 2003, 92, 2140–2143. [Google Scholar] [CrossRef]
- Miranda, C.; Pérez-Rodríguez, Z.; Hernández-Armengol, R.; Quiñones-García, Y.; Betancourt-Purón, T.; Cabrera-Pérez, M.Á. Biowaiver or Bioequivalence: Ambiguity in Sildenafil Citrate BCS Classification. AAPS PharmSciTech 2018, 19, 1693–1698. [Google Scholar] [CrossRef]
- Knapik-kowalczuk, J.; Chmiel, K.; Pacułt, J.; Bialek, K.; Tajber, L.; Paluch, M. Enhancement of the physical stability of amorphous sildenafil in a binary mixture, with either a plasticizing or antiplasticizing compound. Pharmaceutics 2020, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Barbas, R.; Font-Bardia, M.; Prohens, R. Polymorphism of Sildenafil: A New Metastable Desolvate. Cryst. Growth Des. 2018, 18, 3740–3746. [Google Scholar] [CrossRef]
- Stepanovs, D.; Mishnev, A. Molecular and crystal structure of sildenafil base. Z. Nat.-Sect. B J. Chem. Sci. 2012, 67, 491–494. [Google Scholar] [CrossRef]
- Patent Zegarac, M.; Mestrovic, E.; Dumbovic, A.; Devcic, M.; Tudja, P. Pharmaceutically Acceptable Cocrystalline Forms of Sildenafil. WO Patent WO2007110559A1, 4 October 2007. [Google Scholar]
- Jung, S.Y.; Seo, Y.G.; Kim, G.K.; Woo, J.S.; Yong, C.S.; Choi, H.G. Comparison of the solubility and pharmacokinetics of sildenafil salts. Arch. Pharm. Res. 2011, 34, 451–454. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kim, D.W.; Seo, Y.G.; Woo, J.S.; Yong, C.S.; Choi, H.G. Development of sildenafil-loaded orally disintegrating tablet with new lactate salt. Drug Dev. Ind. Pharm. 2011, 38, 635–641. [Google Scholar] [CrossRef]
- Sanphui, P.; Tothadi, S.; Ganguly, S.; Desiraju, G.R. Salt and cocrystals of sildenafil with dicarboxylic acids: Solubility and pharmacokinetic advantage of the glutarate salt. Mol. Pharm. 2013, 10, 4687–4697. [Google Scholar] [CrossRef]
- Žegarac, M.; Lekšić, E.; Šket, P.; Plavec, J.; Bogdanović, M.D.; Bučar, D.-K.; Dumić, M.; Meštrović, E. A sildenafil cocrystal based on acetylsalicylic acid exhibits an enhanced intrinsic dissolution rate. CrystEngComm 2014, 16, 32–35. [Google Scholar] [CrossRef]
- Stepanovs, D.; Jure, M.; Mishnev, A. Preparation and crystal structure of sildenafil salicylate. Mendeleev Commun. 2015, 25, 49–50. [Google Scholar] [CrossRef]
- Barbas, R.; Kumar, V.; Vallcorba, O.; Prohens, R.; Frontera, A. Sildenafil–resorcinol cocrystal: XRPD structure and DFT calculations. Crystals 2020, 10, 1126. [Google Scholar] [CrossRef]
- Banerjee, R.; Bhatt, P.M.; Desiraju, G.R. Solvates of sildenafil saccharinate. A new host material. Cryst. Growth Des. 2006, 6, 1468–1478. [Google Scholar] [CrossRef]
- Barbas, R.; Font-Bardia, M.; Paradkar, A.; Hunter, C.A.; Prohens, R. Combined Virtual/Experimental Multicomponent Solid Forms Screening of Sildenafil: New Salts, Cocrystals, and Hybrid Salt-Cocrystals. Cryst. Growth Des. 2018, 18, 7618–7627. [Google Scholar] [CrossRef]
- Elshafeey, A.H.; Bendas, E.R.; Mohamed, O.H. Intranasal microemulsion of sildenafil citrate: In vitro evaluation and in vivo pharmacokinetic study in rabbits. AAPS PharmSciTech 2009, 10, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chow, M.S.S.; Zuo, Z. Mechanistic analysis of pH-dependent solubility and trans-membrane permeability of amphoteric compounds: Application to sildenafil. Int. J. Pharm. 2008, 352, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Gobry, V.; Bouchard, G.; Carrupt, P.A.; Testa, B.; Girault, H.H. Physicochemical characterization of sildenafil: Ionization, lipophilicity behavior, and ionic-partition diagram studied by two-phase titration and electrochemistry. Helv. Chim. Acta 2000, 83, 1465–1474. [Google Scholar] [CrossRef]
- Liaw, J.; Chang, T.W. Determination of transdermal sildenafil in nude mouse skin by reversed-phase high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 2001, 765, 161–166. [Google Scholar] [CrossRef]
- Simiele, M.; Pensi, D.; Pasero, D.; Ivaldi, F.; Rinaldi, M.; Di Perri, G.; Ranieri, V.M.; D’Avolio, A. Development and validation of an ultra performance liquid chromatography tandem mass method for sildenafil and N-desmethyl sildenafil plasma determination and quantification. J. Chromatogr. B 2015, 1001, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Akula, P.; Lakshmi, P.K. Effect of pH on weakly acidic and basic model drugs and determination of their ex vivo transdermal permeation routes. Braz. J. Pharm. Sci. 2018, 54, 70. [Google Scholar] [CrossRef]
- Júlio, T.A.; Zâmara, I.F.; Garcia, J.S.; Trevisan, M.G. Compatibility of sildenafil citrate and pharmaceutical excipients by thermal analysis and LC-UV. J. Therm. Anal. Calorim. 2013, 111, 2037–2044. [Google Scholar] [CrossRef]
- Canbay, H.S.; Doğantürk, M. Compatibility Studies of Sildenafil with Different Excipients by Using TGA, DSC, XRD and FTIR. Celal Bayar Üniversitesi Fen Bilim. Derg. 2019, 15, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Badwan, A.A.; Nabuls, L.; Al-Omari, M.M.; Daraghmeh, N.; Ashour, M. Sildenafil Citrate. In Analytical Profiles of Drug Substances and Excipients; EMA (European Medical Agency): London, UK, 2001; Volume 27, pp. 339–376. [Google Scholar]
- Sawatdee, S.; Pakawatchai, C.; Nitichai, K.; Srichana, T.; Phetmung, H. Why sildenafil and sildenafil citrate monohydrate crystals are not stable? Saudi Pharm. J. 2015, 23, 504–514. [Google Scholar] [CrossRef] [Green Version]
- Mateescu, C.; Popescu, A.M.; Radu, G.L.; Onisei, T.; Raducanu, A.E. Spectroscopic and spectrometric methods used for the screening of certain herbal food supplements suspected of adulteration. Adv. Pharm. Bull. 2017, 7, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Nugroho, A.; Febriana, Y.; Septiwi, M.; Pratiwi, D.A. Rapid analysis of adulterated sildenafil citrate in marketed herbal aphrodisiacs using infrared spectroscopy. AIP Conf. Proc. 2018, 2026, 020003. [Google Scholar] [CrossRef]
- Neto, J.C.; Lisboa, F.L.C. ATR-FTIR characterization of generic brand-named and counterfeit sildenafil- and tadalafil-based tablets found on the Brazilian market. Sci. Justice 2017, 57, 283–295. [Google Scholar] [CrossRef]
- Sacré, P.-Y.; Deconinck, E.; De Beer, T.; Courselle, P.; Vancauwenberghe, R.; Chiap, P.; Crommen, J.; De Beer, J.O. Comparison and combination of spectroscopic techniques for the detection of counterfeit medicines. J. Pharm. Biomed. Anal. 2010, 53, 445–453. [Google Scholar] [CrossRef] [Green Version]
- Yathirajan, H.S.; Nagaraj, B.; Nagaraja, P.; Bolte, M. Sildenafil citrate monohydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, 489–491. [Google Scholar] [CrossRef]
- Wawer, I.; Pisklak, M.; Chilmonczyk, Z. 1H, 13C, 15N NMR analysis of sildenafil base and citrate (Viagra) in solution, solid state and pharmaceutical dosage forms. J. Pharm. Biomed. Anal. 2005, 38, 865–870. [Google Scholar] [CrossRef]
- Abraham, A.; Apperley, D.C.; Byard, S.J.; Ilott, A.J.; Robbins, A.J.; Zorin, V.; Harris, R.K.; Hodgkinson, P. Characterising the role of water in sildenafil citrate by NMR crystallography. CrystEngComm 2016, 18, 1054–1063. [Google Scholar] [CrossRef] [Green Version]
- McMahon, C.G.; Samali, R.; Johnson, H. Efficacy, safety and patient acceptance of sildenafil citrate as treatment for erectile dysfunction. J. Urol. 2000, 164, 1192–1196. [Google Scholar] [CrossRef]
- Rosen, R.C.; Kostis, J.B. Overview of phosphodiesterase 5 inhibition in erectile dysfunction. Am. J. Cardiol. 2003, 92, 9–18. [Google Scholar] [CrossRef]
- Bell, A.; Davis, M.; Close, F.; Honeywell, M.; Branch, E. Sildenafil citrate (revatio) for the treatment of pulmonary arterial hypertension. Conn. Med. 2006, 6, 381–383. [Google Scholar]
- Maclean, M.R.; Johnston, E.D.; Mcculloch, K.M.; Pooley, L.; Houslay, M.D.; Sweeney, G. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: Changes in pulmonary hypertension. J. Pharmacol. Exp. Ther. 1997, 283, 619–624. [Google Scholar]
- Raja, S.G.; Danton, M.D.; MacArthur, K.J.; Pollock, J.C. Treatment of Pulmonary Arterial Hypertension with Sildenafil: From Pathophysiology to Clinical Evidence. J. Cardiothorac. Vasc. Anesth. 2006, 20, 722–735. [Google Scholar] [CrossRef]
- Isidori, A.M.; Giannetta, E.; Pofi, R.; Venneri, M.A.; Gianfrilli, D.; Campolo, F.; Mastroianni, C.M.; Lenzi, A.; d’Ettorre, G. Targeting the NO-cGMP-PDE5 pathway in COVID-19 infection. The DEDALO project. Andrology 2020, 9, 33–38. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhang, H.; Ji, H.F.; Chen, Q. Computational view toward the inhibition of SARS-CoV-2 spike glycoprotein and the 3CL protease. Computation 2020, 9, 53. [Google Scholar] [CrossRef]
- Islam, B.N.; Sharman, S.K.; Hou, Y.; Bridges, A.E.; Singh, N.; Kim, S.; Kolhe, R.; Trillo-Tinoco, J.; Rodriguez, P.C.; Berger, F.G.; et al. Sildenafil suppresses inflammation-driven colorectal cancer in mice. Cancer Prev. Res. 2017, 10, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Mei, X.-L.; Yang, Y.; Zhang, Y.-J.; Li, Y.; Zhao, J.-M.; Qiu, J.-G.; Zhang, W.-J.; Jiang, Q.-W.; Xue, Y.-Q.; Zheng, D.-W.; et al. Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. Am. J. Cancer Res. 2015, 5, 3311–3324. [Google Scholar]
- Dent, P.; Booth, L.; Roberts, J.L.; Poklepovic, A.; Hancock, J.F. (Curcumin+sildenafil) enhances the efficacy of 5FU and anti-PD1 therapies in vivo. J. Cell. Physiol. 2020, 235, 6862–6874. [Google Scholar] [CrossRef] [PubMed]
- Sarfati, M.; Mateo, V.; Baudet, S.; Rubio, M.; Fernandez, C.; Davi, F.; Binet, J.-L.; Delic, J.; Merle-Béral, H. Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood 2003, 101, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Durrant, D.; Mitchell, C.; Mayton, E.; Hoke, N.N.; Salloum, F.N.; Park, M.A.; Qureshi, I.; Lee, R.; Dent, P.; et al. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc. Natl. Acad. Sci. USA 2010, 107, 18202–18207. [Google Scholar] [CrossRef] [Green Version]
- Di, X.; Gennings, C.; Bear, H.D.; Graham, L.J.; Sheth, C.M.; White, K.L.; Gewirtz, D.A. Influence of the phosphodiesterase-5 inhibitor, sildenafil, on sensitivity to chemotherapy in breast tumor cells. Breast Cancer Res. Treat. 2010, 124, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Booth, L.; Roberts, J.L.; Cruickshanks, N.; Conley, A.; Durrant, D.E.; Das, A.; Fisher, P.B.; Kukreja, R.C.; Grant, S.; Poklepovic, A.; et al. Phosphodiesterase 5 inhibitors enhance chemotherapy killing in gastrointestinal/genitourinary cancer cells. Mol. Pharmacol. 2014, 85, 408–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grover-Páez, F.; Rivera, G.V.; Ortíz, R.G. Sildenafil citrate diminishes microalbuminuria and the percentage of A1c in male patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2007, 78, 136–140. [Google Scholar] [CrossRef]
- Aversa, A.; Vitale, C.; Volterrani, M.; Fabbri, A.; Spera, G.; Fini, M.; Rosano, G.M.C. Chronic administration of Sildenafil improves markers of endothelial function in men with Type 2 diabetes. Diabet. Med. 2008, 25, 37–44. [Google Scholar] [CrossRef]
- Mandosi, E.; Giannetta, E.; Filardi, T.; Lococo, M.; Bertolini, C.; Fallarino, M.; Gianfrilli, D.; Venneri, M.A.; Lenti, L.; Lenzi, A.; et al. Endothelial dysfunction markers as a therapeutic target for Sildenafil treatment and effects on metabolic control in type 2 diabetes. Expert Opin. Ther. Targets 2015, 19, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.J.; Muirhead, G.J.; Harness, J.A. Pharmacokinetics of sildenafil citrate after single oral doses in healthy male subjects: Absolute bioavailability, food effects and dose proportionality. Br. J. Clin. Pharmacol. 2002, 53, 5S–12S. [Google Scholar] [CrossRef] [Green Version]
- Muirhead, G.J.; Rance, D.J.; Walker, D.K.; Wastall, P. Comparative human pharmacokinetics and metabolism of single-dose oral and intravenous sildenafil citrate. Br. J. Clin. Pharmacol. Suppl. 2002, 53, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ballard, S.A.; Gingell, C.J.; Tang, K.; Turner, L.A.; Price, M.E.; Naylor, A.M. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J. Urol. 1998, 159, 2164–2171. [Google Scholar] [CrossRef]
- Zusman, R.M.; Morales, A.; Glasser, D.B.; Osterloh, I.H. Overall cardiovascular profile of sildenafil citrate. Am. J. Cardiol. 1999, 83, 35–44. [Google Scholar] [CrossRef]
- Jackson, G.; Benjamin, N.; Jackson, N.; Allen, M.J. Effects of sildenafil citrate on human hemodynamics. Am. J. Cardiol. 1999, 83, 13–20. [Google Scholar] [CrossRef]
- Moreland, R.B.; Goldstein, I.; Kim, N.N.; Traish, A. Sildenafil citrate, a selective phosphodiesterase type 5 inhibitor: Research and clinical implications in erectile dysfunction. Trends Endocrinol. Metab. 1999, 10, 97–104. [Google Scholar] [CrossRef]
- Gingell, C.; Sultana, S.R.; Wulff, M.B.; Gepi-Attee, S. Duration of action of sildenafil citrate in men with erectile dysfunction. J. Sex. Med. 2004, 1, 179–184. [Google Scholar] [CrossRef]
- Moncada, I.; Jara, J.; Subirá, D.; Castaño, I.; Hernández, C. Efficacy of sildenafil citrate at 12 h after dosing: Re-exploring the therapeutic window. Eur. Urol. 2004, 46, 357–361. [Google Scholar] [CrossRef]
- Steidle, C.P.; McCullough, A.R.; Kaminetsky, J.C.; Crowley, A.R.; Siegel, R.L.; Deriesthal, H.; Tseng, L.-J. Early sildenafil dose optimization and personalized instruction improves the frequency, flexibility, and success of sexual intercourse in men with erectile dysfunction. Int. J. Impot. Res. 2007, 19, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Francis, S.H. Sildenafil: Efficacy, safety, tolerability and mechanism of action in treating erectile dysfunction. Expert Opin. Drug Metab. Toxicol. 2005, 1, 283–293. [Google Scholar] [CrossRef]
- Blount, M.A.; Zoraghi, R.; Bessay, E.P.; Beasley, A.; Francis, S.H.; Corbin, J.D. Conversion of phosphodiesterase-5 (PDE5) catalytic site to higher affinity by PDE5 inhibitors. J. Pharmacol. Exp. Ther. 2007, 323, 730–737. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Kovar, A.; Meibohm, B. The clinical pharmacokinetics of phosphodiesterase-5 inhibitors for erectile dysfunction. J. Clin. Pharmacol. 2005, 45, 987–1003. [Google Scholar] [CrossRef]
- Rybalkina, I.G.; Tang, X.B.; Rybalkin, S.D. Multiple affinity states of cGMP-specific phosphodiesterase for sildenafil inhibition defined by cGMP-dependent and cGMP-independent mechanisms. Mol. Pharmacol. 2010, 77, 670–677. [Google Scholar] [CrossRef] [Green Version]
- Corbin, J.D.; Blount, M.A.; Weeks, J.L.; Beasley, A.; Kuhn, K.P.; Ho, Y.S.J.; Saidi, L.F.; Hurley, J.H.; Kotera, J.; Francis, S.H. [3H]sildenafil binding to phosphodiesterase-5 is specific, kinetically heterogeneous, and stimulated by cGMP. Mol. Pharmacol. 2003, 63, 1364–1372. [Google Scholar] [CrossRef]
- Human medicines European public assessment report (EPAR): Viagra, sildenafil, Erectile Dysfunction, Date of authorisation: 13/09/1998, Revision: 33, Status: Authorised. Case Med. Res. 2019, 25–76.
- Oliver, J.J.; Dear, J.W.; Webb, D.J. Clinical potential of combined organic nitrate and phosphodiesterase type 5 inhibitor in treatment-resistant hypertension. Hypertension 2010, 56, 62–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muirhead, G.J.; Harness, J.A.; Taubel, J. The effects of steady-state erythromycin and azithromycin on the pharmacokinetics of sildenafil citrate in healthy volunteers. Br. J. Clin. Pharmacol. 2002, 53, 37S–43S. [Google Scholar] [CrossRef] [Green Version]
- Muirhead, G.J.; Wulff, M.B.; Fielding, A.; Kleinermans, D.; Buss, N. Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir. Br. J. Clin. Pharmacol. 2000, 50, 99–107. [Google Scholar] [CrossRef]
- Sekar, V.; Lefebvre, E.; De Marez, T.; De Pauw, M.; De Paepe, E.; Vangeneugden, T.; Hoetelmans, R.M.W. Effect of Repeated Doses of Darunavir plus Low-Dose Ritonavir on the Pharmacokinetics of Sildenafil in Healthy Male Subjects. Clin. Drug Investig. 2008, 28, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Merry, C.; Barry, M.G.; Ryan, M.; Tjia, J.F.; Hennessy, M.; Eagling, V.A.; Mulcahy, F.; Back, D.J. Interaction of sildenafil and indinavir when co-administered to HIV-positive patients. AIDS 1999, 13, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Bratt, G.; Ståhle, L. Sildenafil does not alter nelfinavir pharmacokinetics. Ther. Drug Monit. 2003, 25, 240–242. [Google Scholar] [CrossRef]
- Wilner, K.; Laboy, L.; LeBel, M. The effects of cimetidine and antacid on the pharmacokinetic profile of sildenafil citrate in healthy male volunteers. Br. J. Clin. Pharmacol. 2002, 53, 31S–36S. [Google Scholar] [CrossRef] [Green Version]
- Hesse, C.; Siedler, H.; Burhenne, J.; Riedel, K.D.; Haefeli, W.E. Fluvoxamine affects sildenafil kinetics and dynamics. J. Clin. Psychopharmacol. 2005, 25, 589–592. [Google Scholar] [CrossRef]
- Jetter, A.; Kinzig-Schippers, M.; Walchner-Bonjean, M.; Hering, U.; Bulitta, J.; Schreiner, P.; Sörgel, F.; Fuhr, U. Effects of grapefruit juice on the pharmacokinetics of sildenafil. Clin. Pharmacol. Ther. 2002, 71, 21–29. [Google Scholar] [CrossRef]
- Al-Ghazawi, M.A.; Tutunji, M.S.; Aburuz, S.M. The effects of pummelo juice on pharmacokinetics of sildenafil in healthy adult male Jordanian volunteers. Eur. J. Clin. Pharmacol. 2009, 66, 159–163. [Google Scholar] [CrossRef]
- Paul, G.A.; Gibbs, J.S.R.; Boobis, A.R.; Abbas, A.; Wilkins, M.R. Bosentan decreases the plasma concentration of sildenafil when coprescribed in pulmonary hypertension. Br. J. Clin. Pharmacol. 2005, 60, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Burgess, G.; Hoogkamer, H.; Collings, L.; Dingemanse, J. Mutual pharmacokinetic interactions between steady-state bosentan and sildenafil. Eur. J. Clin. Pharmacol. 2008, 64, 43–50. [Google Scholar] [CrossRef]
- Treiber, A.; Schneiter, R.; Häusler, S.; Stieger, B. Bosentan is a substrate of human OATP1B1 and OATP1B3: Inhibition of hepatic uptake as the common mechanism of its interactions with cyclosporin A, rifampicin, and sildenafil. Drug Metab. Dispos. 2007, 35, 1400–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidharta, P.N.; van Giersbergen, P.L.M.; Wolzt, M.; Dingemanse, J. Investigation of mutual pharmacokinetic interactions between macitentan, a novel endothelin receptor antagonist, and sildenafil in healthy subjects. Br. J. Clin. Pharmacol. 2014, 78, 1035–1042. [Google Scholar] [CrossRef] [Green Version]
- Spence, R.; Mandagere, A.; Dufton, C.; Venitz, J. Pharmacokinetics and safety of ambrisentan in combination with sildenafil in healthy volunteers. J. Clin. Pharmacol. 2008, 48, 1451–1459. [Google Scholar] [CrossRef]
- Muirhead, G.J.; Wilner, K.; Colburn, W.; Haug-Pihale, G.; Rouviex, B. The effects of age and renal and hepatic impairment on the pharmacokinetics of sildenafil citrate. Br. J. Clin. Pharmacol. 2002, 53, 21S–30S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossman, E.B.; Swan, S.K.; Muirhead, G.J.; Gaffney, M.; Chung, M.; Deriesthal, H.; Chow, D.; Raij, L. The pharmacokinetics and hemodynamics of sildenafil citrate in male hemodialysis patients. Kidney Int. 2004, 66, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Discussion, S.; Fosavance, I.; Pfizer Ltd. Summary of Product Characteristics: Revatio® 20 mg Film-Coated Tablets. 2007. Available online: http://www.emea.europa.eu/humandocs/PDFs/EPAR/Revatio/H-638-PI-en.pdf (accessed on 10 September 2007).
- Barst, R.J.; Ivy, D.D.; Gaitan, G.; Szatmari, A.; Rudzinski, A.; Garcia, A.E.; Sastry, B.; Pulido, T.; Layton, G.R.; Serdarevic-Pehar, M.; et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation 2012, 125, 324–334. [Google Scholar] [CrossRef]
- Dodgen, A.L.; Hill, K.D. Safety and tolerability considerations in the use of sildenafil for children with pulmonary arterial hypertension. Drug. Healthc. Patient Saf. 2015, 7, 175–183. [Google Scholar]
- Mukherjee, A.; Dombi, T.; Wittke, B.; Lalonde, R. Population pharmacokinetics of sildenafil in term neonates: Evidence of rapid maturation of metabolic clearance in the early postnatal period. Clin. Pharmacol. Ther. 2009, 85, 56–63. [Google Scholar] [CrossRef]
- Dey, P.; Maiti, S. Orodispersible tablets: A new trend in drug delivery. J. Nat. Sci. Biol. Med. 2010, 1, 2–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocci, A.; Capece, M.; Cito, G.; Russo, G.I.; Falcone, M.; Timpano, M.; Rizzo, M.; Camera, P.A.D.; Morselli, S.; Campi, R.; et al. Effectiveness and Safety of Oro-Dispersible Sildenafil in a New Film Formulation for the Treatment of Erectile Dysfunction: Comparison between Sildenafil 100-mg Film-Coated Tablet and 75-mg Oro-Dispersible Film. J. Sex. Med. 2017, 14, 1606–1611. [Google Scholar] [CrossRef]
- Sheu, M.T.; Hsieh, C.M.; Chen, R.N.; Chou, P.Y.; Ho, H.O. Rapid-Onset Sildenafil Sublingual Drug Delivery Systems: In Vitro Evaluation and In Vivo Pharmacokinetic Studies in Rabbits. J. Pharm. Sci. 2016, 105, 2774–2781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, H.; Cho, S.M.; Choi, Y.W.; Lee, H.J.; Kwon, J.-H.; Kim, S.-W.; Kim, J.W.; Lee, S.; Hong, J.-H. Comparison of pharmacokinetic characteristics of sildenafil citrate chewable tablets and film-coated tablets in healthy male subjects. Transl. Clin. Pharmacol. 2017, 25, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Sawatdee, S.; Atipairin, A.; Yoon, A.S.; Srichana, T.; Changsan, N.; Suwandecha, T.; Chanthorn, W.; Phoem, A. Oral bioavailability and pharmacokinetics of sildenafil citrate dry foam tablets in rats. Cogent Med. 2018, 5. [Google Scholar] [CrossRef]
- Hussain, A.A.; Dittert, L.W.; Traboulsi, A. Nasal Administration of Sildenafil for the Treatment of Erectile Dysfunction. 1998. Available online: https://patents.google.com/patent/WO1999066933A1/en (accessed on 12 April 2021).
- Elnaggar, Y.S.R.; El-Massik, M.A.; Abdallah, O.Y. Fabrication, appraisal, and transdermal permeation of sildenafil citrate-loaded nanostructured lipid carriers versus solid lipid nanoparticles. Int. J. Nanomed. 2011, 6, 3195–3205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; He, W.; Ye, L.; Zhu, Y.; Tian, Y.; Chen, L.; Yang, J.; Miao, M.; Shi, Y.; Azevedo, H.S.; et al. Targeted delivery of sildenafil for inhibiting pulmonary vascular remodeling. Hypertension 2019, 73, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, A.; Kiyota, T. A physiologically-based drug absorption modeling for orally disintegrating tablets. Eur. J. Pharm. Biopharm. 2020, 152, 1–9. [Google Scholar] [CrossRef]
- Damle, B.; Duczynski, G.; Jeffers, B.W.; Crownover, P.; Coupe, A.; Labadie, R.R. Pharmacokinetics of a novel orodispersible tablet of sildenafil in healthy subjects. Clin. Ther. 2014, 36, 236–244. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, S.K.; Tripathi, P.K.; Verma, N.K. Formulation and evaluation of fast dissolving tablet of sildenafil citrate. Der Pharm. Lett. 2014, 6, 56–62. [Google Scholar]
- Lv, Y.; Luo, B.; Labadie, R.R.; Zhu, H.; Feng, Y.; Ernst, C.; Crownover, P.H.; Liang, Y.; Zhao, Q. Bioequivalence and Bioavailability of an Orodispersible Tablet of Sildenafil Citrate in Healthy Chinese Male Subjects. Clin. Pharmacol. Drug Dev. 2020, 9, 573–581. [Google Scholar] [CrossRef]
- Wang, C.; Sun, C.C. The efficient development of a sildenafil orally disintegrating tablet using a material sparing and expedited approach. Int. J. Pharm. 2020, 589, 119816. [Google Scholar] [CrossRef]
- Loprete, L.; Leuratti, C.; Frangione, V.; Radicioni, M. Pharmacokinetics of a Novel Sildenafil Orodispersible Film Administered by the Supralingual and the Sublingual Route to Healthy Men. Clin. Drug Investig. 2018, 38, 765–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radicioni, M.; Castiglioni, C.; Giori, A.; Cupone, I.; Frangione, V.; Rovati, S. Bioequivalence study of a new sildenafil 100 mg orodispersible film compared to the conventional film-coated 100 mg tablet administered to healthy male volunteers. Drug Des. Devel. Ther. 2017, 11, 1183–1192. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.; Van Riet-Nales, D.; Hermans, E.; De Vries, R.; Hilton, G.; Blowers, P.; Salunke, S. European Paediatric Formulation Initiative workshop report: Improving the administration of oral liquid medicines in paediatrics using dosing syringes and enteral accessories. Eur. J. Pharm. Biopharm. 2020, 151, 91–97. [Google Scholar] [CrossRef]
- Provenza, N.; Calpena, A.C.; Mallandrich, M.; Halbaut, L.; Clares, B. Design and physicochemical stability studies of paediatric oral formulations of sildenafil. Int. J. Pharm. 2014, 460, 234–239. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Ndongo, M.N.; Checchio, T.M.; Cook, J.; Duncan, B.; Labadie, R.R. A randomized, open-label 3-way crossover study to investigate the relative bioavailability and bioequivalence of crushed sildenafil 20mg tablets mixed with apple sauce, extemporaneously prepared suspension (EP), and intact sildenafil 20mg tablets in healt. Clin. Pharmacol. Drug Dev. 2015, 4, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Dischinger, A.; Page, S.; Kleinebudde, P. Fast dissolving fillers in dry foam formulation. Powder Technol. 2015, 270, 494–501. [Google Scholar] [CrossRef]
- Sprunk, A.; Page, S.; Kleinebudde, P. Influence of process parameters and equipment on dry foam formulation properties using indomethacin as model drug. Int. J. Pharm. 2013, 455, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Sawatdee, S.; Atipairin, A.; Yoon, A.S.; Srichana, T. Enhanced dissolution of sildenafil dry foam tablets. Asian J. Pharm. Sci. 2016, 11, 191–192. [Google Scholar] [CrossRef]
- Sawatdee, S.; Atipairin, A.; Yoon, A.S.; Srichana, T.; Changsan, N. Enhanced dissolution of sildenafil citrate as dry foam tablets. Pharm. Dev. Technol. 2019, 24, 1–11. [Google Scholar] [CrossRef]
- Khunt, D.; Polaka, S.; Shrivas, M.; Misra, M. Biodistribution and amyloid beta induced cell line toxicity study of intranasal Rivastigmine microemulsion enriched with Fish Oil and Butter oil. J. Drug Deliv. Sci. Technol. 2020, 57, 101661. [Google Scholar] [CrossRef]
- Lu, H.T.; Chen, R.N.; Sheu, M.T.; Chang, C.C.; Chou, P.Y.; Ho, H.O. Rapid-onset sildenafil nasal spray carried by microemulsion systems: In vitro evaluation and in vivo pharmacokinetic studies in rabbits. Xenobiotica 2011, 41, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Ghofrani, H.A.; Torbicki, A.; Barst, R.J.; Rubin, L.J.; Badesch, D.; Fleming, T.; Parpia, T.; Burgess, G.; Branzi, A.; et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2005, 353, 2148–2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck-Broichsitter, M.; Hecker, A.; Kosanovic, D.; Schmehl, T.; Gessler, T.; Weissmann, N.; Ghofrani, H.A.; Kissel, T.; Seeger, W.; Schermuly, R.T. Prolonged vasodilatory response to nanoencapsulated sildenafil in pulmonary hypertension. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Beck-Broichsitter, M.; Schmehl, T.; Gessler, T.; Seeger, W.; Kissel, T. Development of a biodegradable nanoparticle platform for sildenafil: Formulation optimization by factorial design analysis combined with application of charge-modified branched polyesters. J. Control. Release 2012, 157, 469–477. [Google Scholar] [CrossRef]
- Cevc, G.; Vierl, U. Nanotechnology and the transdermal route. A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Medarević, D.; Ibrić, S.; Vardaka, E.; Mitrić, M.; Nikolakakis, I.; Kachrimanis, K. Insight into the Formation of Glimepiride Nanocrystals by Wet Media Milling. Pharmaceutics 2020, 12, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouranidis, A.; Gkampelis, N.; Vardaka, E.; Karagianni, A.; Tsiptsios, D.; Nikolakakis, I.; Kachrimanis, K. Overcoming the Solubility Barrier of Ibuprofen by the Rational Process Design of a Nanocrystal Formulation. Pharmaceutics 2020, 12, 969. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 1–40. [Google Scholar] [CrossRef]
- EMA. ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS [WWW Document]. 2020. Available online: https://www.ema.europa.eu/en/documents/product-information/sildenafil-actavis-epar-product-information_en.pdf (accessed on 12 April 2021).
- EMA. Annex I Summary of Product Characteristics [WWW Document]. 2014. Available online: https://www.ema.europa.eu/en/documents/product-information/revatio-epar-product-information_en.pdf (accessed on 12 April 2021).
- EMA. Annex I Summary of Product Characteristics [WWW Document]. 2008. Available online: https://www.ema.europa.eu/en/documents/product-information/viagra-epar-product-information_en.pdf (accessed on 12 April 2021).
- Infarmed, Summary Public Assessment Report [WWW Document]. 2018. Available online: https://mri.cts-mrp.eu/human/downloads/PT_H_2013_001_PAR.pdf (accessed on 12 April 2021).
- HMA. Chewable Sildenafil. 2012.
- EMA. Annex I Summary of Product Characteristics. 2010. Available online: https://ec.europa.eu/health/documents/community-register/2010/2010061081029/anx_81029_en.pdf (accessed on 12 April 2021).
- EDQM, European Pharmacopoeia Eighth Edition. EDQM Publ. ID. 2013. Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition (accessed on 12 April 2021).
- Soubra, R.; Gazy, A.A.; Saab, M.; Al Jamal, M.K. Identification and Quantification of Phosphodiesterase-5 Inhibitors as Adulterants in Dietary Supplements Marked for Sexual Enhancement in the Lebanese Market. Int. J. Pharm. Pharm. Sci. 2020, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Baokar, S.; Pawar, V.; Patil, R.N.; Jagatap, R.; Ekatpure, N. Validation of simple and rapid UV-spectrophotometric method with stress degradation study for sildenafil citrate. Res. J. Pharm. Technol. 2012, 5, 214–218. [Google Scholar]
- Altıokka, G.; Atkosar, Z.; Sener, E.; Tunçel, M. FIA of sildenafil citrate using UV-detection. J. Pharm. Biomed. Anal. 2001, 25, 339–342. [Google Scholar] [CrossRef]
- Amin, A.S.; El-Beshbeshy, A.M. Utility of Certain σ and π-Acceptors for the Spectrophotometric Determination of Sildenafil Citrate (Viagra). Microchim. Acta 2001, 137, 63–69. [Google Scholar] [CrossRef]
- Dinesh, N. Extractive spectrophotometric methods for the assay of sildenafil citrate (Viagra) in pure form and in pharmaceutical formulations. Talanta 2002, 57, 757–764. [Google Scholar] [CrossRef]
- Issa, Y.M.; El-Hawary, W.F.; Youssef, A.F.A.; Senosy, A.R. Spectrophotometric determination of sildenafil citrate in pure form and in pharmaceutical formulation using some chromotropic acid azo dyes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 1297–1303. [Google Scholar] [CrossRef]
- Wang, C.C.; Fernandez, L. Spectrofluorimetric Determination of Sildenafil: A New Analytical Alternative for Its Analysis. J. Chin. Med. Res. Dev. 2012, 1, 54–60. [Google Scholar]
- Wang, C.C.; Gómez, R.A.; Fernandez, L.P. Determination of sildenafil by preconcentration on surfactant coated polymeric resin followed by spectrofluorimetry. J. Pharm. Anal. 2013, 3, 173–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, S.; He, Y. Analysis of Sildenafil in Liquor and Health Wine Using Surface Enhanced Raman Spectroscopy. Int. J. Mol. Sci. 2019, 20, 2722. [Google Scholar] [CrossRef] [Green Version]
- Vredenbregt, M.J.; Blok-Tip, L.; Hoogerbrugge, R.; Barends, D.M.; de Kaste, D. Screening suspected counterfeit Viagra® and imitations of Viagra® with near-infrared spectroscopy. J. Pharm. Biomed. Anal. 2006, 40, 840–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Veij, M.; Deneckere, A.; Vandenabeele, P.; de Kaste, D.; Moens, L. Detection of counterfeit Viagra® with Raman spectroscopy. J. Pharm. Biomed. Anal. 2008, 46, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Özkan, S.A.; Uslu, B.; Zuman, P. Electrochemical oxidation of sildenafil citrate (Viagra) on carbon electrodes. Anal. Chim. Acta 2004, 501, 227–233. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Elnemma, E.M.; Mahmoud, W.H.; Mohammed, A.H.K. Continuous potentiometric monitoring of viagra (sildenafil) in pharmaceutical preparations using novel membrane sensors. J. Appl. Electrochem. 2006, 36, 139–146. [Google Scholar] [CrossRef]
- Baranowska, I.; Koper, M.; Markowski, P. Electrochemical determination of carvedilol, sildenafil and paracetamol using glassy carbon electrode. Chem. Anal. 2008, 53, 967–981. [Google Scholar]
- St. Staden, R.-I.; van Staden, J.F.; Aboul-Enein, H.Y. Diamond paste-based electrodes for the determination of sildenafil citrate (Viagra). J. Solid State Electrochem. 2010, 14, 997–1000. [Google Scholar] [CrossRef]
- Farghali, R.A.; Ahmed, R.A. A Novel Electrochemical Sensor for Determination of Sildenafil Citrate (Viagra) in Pure Form and in Biological and Pharmaceutical Formulations. Int. J. Electrochem. Sci. 2012, 7, 3008–13019. [Google Scholar]
- Júnior, A.C.V.L.; Luz, R.d.S.; Damos, F.S.; Santos, A.S.d.; Franco, D.L.; Santos, W.T.P.d. Determination of sildenafil citrate (Viagra®) in various pharmaceutical formulations by flow injection analysis with multiple pulse amperometric detection. J. Braz. Chem. Soc. 2012, 23, 1800–1806. [Google Scholar] [CrossRef] [Green Version]
- Sasal, A.; Tyszczuk-Rotko, K. Screen-printed sensor for determination of sildenafil citrate in pharmaceutical preparations and biological samples. Microchem. J. 2019, 149, 104065. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Hefnawy, M.M. Rapid Determination of Sildenafil Citrate in Pharmaceutical Preparations Using Monolithic Silica HPLC Column. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 2897–2908. [Google Scholar] [CrossRef]
- Daraghmeh, N.; Al-Omari, M.; Badwan, A.; Jaber, A.M. Determinaton of sildenafil citrate and related substances in the commercial products and tablet dosage form using HPLC. J. Pharm. Biomed. Anal. 2001, 25, 483–492. [Google Scholar] [CrossRef]
- Yilmaz, E.; Ulusoy, H.İ.; Demir, Ö.; Soylak, M. A new magnetic nanodiamond/graphene oxide hybrid (Fe3O4@ND@GO) material for pre-concentration and sensitive determination of sildenafil in alleged herbal aphrodisiacs by HPLC-DAD system. J. Chromatogr. B 2018, 1084, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Rajput, P.; Thakkar, A.; Sarma, G.S. RP-HPLC Method Development for Estimation of Sildenafil Citrate in Tablets and in Seminal Fluid. J. Appl. Pharm. Sci. 2012, 2, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Reddy, B.P.K.; Reddy, Y.R. Validation and stability indicating RP-HPLC method for the determination of sildenafil citrate in pharmaceutical formulations and human plasma. E-J. Chem. 2008, 5, 1117–1122. [Google Scholar] [CrossRef]
- Atipairin, A.; Woradechakul, C.; Chee, K.S.; Sawatdee, S.; Yoon, A.S. Method validation for determination of sildenafil citrate in extemporaneous oral suspension. Int. J. Pharm. Pharm. Sci. 2014, 6, 131–136. [Google Scholar]
- Zaharieva, Z.; Tanev, D.; Danalev, D. Development and validation of HPLC/DAD method for simultaneously determination of six prohibited substances in model matrices. Acta Chromatogr. 2020, 32, 276–280. [Google Scholar] [CrossRef]
- EMEA/776768/2009 and EMA/CHMP/633051/2012, CHMP Assessment Report for Sildenafil Teva. 2009. Available online: https://www.ema.europa.eu/en/documents/overview/sildenafil-teva-epar-summary-public_en.pdf (accessed on 12 April 2021).
- ICH Expert Working Group. ICH Guideline Q1A(R2) Stability Testing of New Drug Substances and Products. In International Conference on Harmonization; 2003; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-r2-stability-testing-new-drug-substances-products-step-5_en.pdf (accessed on 12 April 2021).
- Hansen, F.; Øiestad, E.L.; Pedersen-Bjergaard, S. Bioanalysis of pharmaceuticals using liquid-phase microextraction combined with liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2020, 189, 113446. [Google Scholar] [CrossRef]
- Kataoka, H. Pharmaceutical Analysis|Sample Preparation ☆. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Manousi, N.; Tzanavaras, P.D.; Zacharis, C.K. Bioanalytical HPLC Applications of In-Tube Solid Phase Microextraction: A Two-Decade Overview. Molecules 2020, 25, 2096. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Zheng, C.; Fan, M.; Xu, H.; Yi, J.; Feng, Y.; Luo, X.; Li, W.; Cheng, Z. Simultaneous determination and determination of sildenafil and its active metabolite in human plasma using LC–MS/MS method. Biomed. Chromatogr. 2020, 34, e4927. [Google Scholar] [CrossRef]
- Tanaka, S.; Uchida, S.; Hakamata, A.; Miyakawa, S.; Odagiri, K.; Inui, N.; Watanabe, H.; Namiki, N. Simultaneous LC-MS analysis of plasma concentrations of sildenafil, tadalafil, bosentan, ambrisentan, and macitentan in patients with pulmonary arterial hypertension. Pharmazie 2020, 75, 236–239. [Google Scholar]
- Er, E.Ö.; Akkaya, E.; Özbek, B.; Bakırdere, S. A powerful combination of quadruple isotope dilution strategy with dispersive magnetic solid phase extraction for the accurate and precise multi-analyte determination of tadalafil, sildenafil, avanafil and vardenafil in human plasma and urine samples usin. Microchem. J. 2020, 152, 104302. [Google Scholar] [CrossRef]
- Ibrahim, A.E.; Hashem, H.; Elhenawee, M.; Saleh, H. Core–shell particles and monolithic columns; tools for simultaneous LC analysis of avanafil, sildenafil, apomorphine, trazodone, yohimbine, tramadol and dapoxetine in pharmaceutical dosage forms, counterfeit products and human plasma. RSC Adv. 2020, 10, 1379–1387. [Google Scholar] [CrossRef] [Green Version]
- Er, E.Ö.; Akkaya, E.; Özbek, B.; Bakırdere, S. Development of an analytical method based on citric acid coated magnetite nanoparticles assisted dispersive magnetic solid-phase extraction for the enrichment and extraction of sildenafil, tadalafil, vardenafil and avanafil in human plasma and urine prior. Microchem. J. 2019, 147, 269–276. [Google Scholar] [CrossRef]
- Dahshan, H.E.; Helal, M.A.; Mostafa, S.M.; Elgawish, M.S. Development and validation of an HPLC-UV method for simultaneous determination of sildenafil and tramadol in biological fluids: Application to drug-drug interaction study. J. Pharm. Biomed. Anal. 2019, 168, 201–208. [Google Scholar] [CrossRef]
- Er, E.Ö.; Özbek, B.; Bakırdere, S. Accurate and sensitive determination of sildenafil, tadalafil, vardenafil, and avanafil in illicit erectile dysfunction medications and human urine by LC with quadrupole-TOF-MS/MS and their behaviors in simulated gastric conditions. J. Sep. Sci. 2019, 42, 475–483. [Google Scholar]
- Abdulla, M.; Mallah, E.; Abu Dayyih, W.; Abu Rayyan, W.; El-Hajji, F.D.; Bustami, M.; Mansour, K.; Al-Ani, I.; Seder, N.; Arafat, T. Influence of Energy Drinks on Pharmacokinetic Parameters of Sildenafil in Rats. Biomed. Pharmacol. J. 2018, 11, 1317–1328. [Google Scholar] [CrossRef]
- Goffredo, B.M.; Cairoli, S.; Vitale, A.; Corsetti, T.; Pastore, A. Reverse-phase high-performance liquid chromatography for the simultaneous determination of sildenafil and N- desmethyl sildenafil in plasma of children. Biomed. Chromatogr. 2016, 30, 2070–2073. [Google Scholar] [CrossRef]
- Lović, J.; Trišović, N.; Antanasijević, J.; Nikolić, N.D.; Stevanović, S.; Mijin, D.; Vuković, D.; MladenoviĆ, A.; Petrović, S.; Ivić, M.A. Electrochemical determination of sildenafil citrate as standard, in tablets and spiked with human serum at gold and cystein modified gold electrode. J. Electroanal. Chem. 2016, 782, 103–107. [Google Scholar] [CrossRef]
- Rashid, J.; Ahsan, F. A highly sensitive LC–MS/MS method for concurrent determination of sildenafil and rosiglitazone in rat plasma. J. Pharm. Biomed. Anal. 2016, 129, 21–27. [Google Scholar] [CrossRef]
- Al-Hroub, H.; Alkhawaja, B.; Alkhawaja, E.; Arafat, T. Sensitive and rapid HPLC-UV method with back-extraction step for the determination of sildenafil in human plasma. J. Chromatogr. B 2016, 1009–1010, 1–6. [Google Scholar] [CrossRef]
- Strach, B.; Wyska, E.; Pociecha, K.; Krupa, A.; Jachowicz, R. Sensitive and precise HPLC method with back-extraction clean-up step for the determination of sildenafil in rat plasma and its application to a pharmacokinetic study. Biomed. Chromatogr. 2015, 29, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, B.; Kim, J.; In, S.; Baeck, S.; Oh, S.M.; Chung, K.H. An LC–MS/MS method for the determination of five erectile dysfunction drugs and their selected metabolites in hair. J. Chromatogr. B 2015, 978–979, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Farghali, R.A.; Ahmed, R.A. Gold nanoparticles-modified screen-printed carbon electrode for voltammetric determination of sildenafil citrate (Viagra) in pure form, biological and pharmaceutical formulations. Int. J. Electrochem. Sci. 2015, 10, 1494–1505. [Google Scholar]
- Pérez, J.; Pacheco, J.L.C. A Reliable Method to Quantify Sildenafil and its Metabolite N-Demethylsildenafil by HPLC in Plasma of Children. Drug Res. (Stuttg) 2013, 63, 473–476. [Google Scholar]
- Xiao, C.; Tang, M.; Li, J.; Yin, C.C.-R.; Xiang, G.; Xu, L. Determination of sildenafil, vardenafil and aildenafil in human plasma by dispersive liquid–liquid microextraction-back extraction based on ionic liquid and high performance liquid chromatography-ultraviolet detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 931, 111–116. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Purkait, M.K. Novel synthesis of nanocomposite for the extraction of Sildenafil Citrate (Viagra) from water and urine samples: Process screening and optimization. Ultrason. Sonochem. 2017, 38, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Venhuis, B.J.; de Kaste, D. Towards a decade of detecting new analogues of sildenafil, tadalafil and vardenafil in food supplements: A history, analytical aspects and health risks. J. Pharm. Biomed. Anal. 2012, 69, 196–208. [Google Scholar] [CrossRef]
- Li, L.; Low, M.-Y.; Aliwarga, F.; Teo, J.; Ge, X.-W.; Zeng, Y.; Bloodworth, B.; Koh, H.-L. Isolation and identification of hydroxythiohomosildenafil in herbal dietary supplements sold as sexual performance enhancement products. Food Addit. Contam. Part A 2009, 26, 145–151. [Google Scholar] [CrossRef]
- Low, M.-Y.; Zeng, Y.; Li, L.; Ge, X.-W.; Lee, R.; Bloodworth, B.-C.; Koh, H.-L.; Koh, A.P.H.-L. Safety and Quality Assessment of 175 Illegal Sexual Enhancement Products Seized in Red-Light Districts in Singapore. Drug Saf. 2009, 32, 1141–1146. [Google Scholar] [CrossRef]
- Li, W.; Tse, F.L.S. Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Biomed. Chromatogr. 2010, 24, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, H.; Gauri, S.; Rathee, P.; Kumar, V. Development and optimization of fast dissolving oro-dispersible films of granisetron HCl using Box–Behnken statistical design. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 193–201. [Google Scholar] [CrossRef] [Green Version]



| Pharmaceutical Compositions | Ref. | |
|---|---|---|
| sildenafil hydrochloride sildenafil hydrogensulphate sildenafil hemisulphate sildenafil hemitratrate sildenafil esylate sildenafil fumarate | Salts | [17] |
| sildenafil-ACNI sildenafil ACNII | Solvates | [15] |
| sildenafil lactate | [18,19] | |
| sildenafil saccharinate CH3CN sildenafil oxalate sildenafil fumarate trihydrate sildenafil succinate sildenafil glutarate sildenafil adipic acid sildenafil pimelic acid sildenafil suberic acid sildenafil sebacic acid | Solvate Salt Salt Salt Salt Cocrystal Cocrystal Cocrystal Cocrystal | [20] |
| Sildenafil-acetylsalicylic-acid | Cocrystal | [21] |
| sildenafil-salicylic acid | Cocrystal | [22] |
| sildenafil resorcinol | Cocrystal | [23] |
| sildenafil Sacharinate with: CH3NO2, CH3CN, HCONH2, HOC2H4OH, C4H8O2 | Solvates of salts | [24] |
| sildenafil-quercetin sildenafil-3,4-dihydroxybenzoic acid sildenafil-resorcinol sildenafil-tartaric acid sildenafil-caffeic acid sildenafil-methyl gallate sildenafil-3-hydroxybenzoic acid | Cocrystals | [25] |
| Tablets | -Stability -Accurate Dosing -Easy Manufacturing -Small Packaging Size -Easy Handling | -Poor Patient Compliance Due to Swallowing Difficulties -First Pass Metabolism |
|---|---|---|
| Orally disintegrating tablet /film | -disintegration friendly, in the oral cavity, limited volume of saliva -no need of water -easy self-administration | -bitter taste |
| Sublingual tablets | - first past metabolism elimination | |
| Chewable tablets | -easy swallowing -easy self-administration | -first pass metabolism |
| Dry foam tablets | - bioavailability enhancement | -poor patient compliance due to swallowing difficulties -first pass metabolism |
| Intranasal | - first pass metabolism elimination | -poor permeability across nasal mucosa -mucociliary clearance |
| Inhalable | -local action, first pass metabolism elimination | -rapid clearance from the pulmonary compartment |
| Transdermal | -controlled released - first pass metabolism elimination -easy self-administration -avoidance of efflux transporters -improved patient compliance -avoidance of gastrointestinal harsh environment - reduction of dosing frequency -stable plasma levels -extended duration of action | slow absorption rate |
| Intravenous | -highest bioavailability and Cmax | -difficult, demanding process - needle may be painful being the source of infectious disease |
| Formulation | Route | Dose (mg), (mg/mL) | Excipients | Condition | Name | Market Holder | Authorization Date | EPAR |
|---|---|---|---|---|---|---|---|---|
| Film-coated tablets | oral | 25, 50, 100 | Microcrystalline cellulose, Silica, hydrophobic colloidal Croscarmellose sodium, Magnesium stearate Indigo carmine aluminum lake (E132), Sucralose Mannitol, Crospovidone, Polyvinyl acetate, Povidone Flavoring contains: Maltodextrin, Dextrin Natural flavoring contains: Maltodextrin, Glycerol (E422), Propylene glycol (E1520), Lemon flavoring contains: Maltodextrin, Alpha-tocopherol (E307) | ED | Viagra | Pfizer | 1998 | [127] |
| Film-coated tablets | oral | 25, 50, 100 | Tablet core Microcrystalline cellulose, Calcium hydrogen phosphate Croscarmellose sodium, Magnesium stearate Film-coat, Poly(vinyl alcohol), Titanium dioxide (E171) Macrogol 3350, Talc | ED | Sildenafil Teva | Teva | 2009 | [128] |
| Orodispersible tablets | oral | 25, 50, 100 | Hydroxypropyl cellulose (E463), Mannitol (E421) Aspartame (E951), Neohesperidin-dihydrochalcone (E959), Spearmint oil, Peppermint oil (containing sorbitol (E420)), Crospovidone, Calcium silicate, Magnesium stearate (E572) | ED | Vizarsin | Krka d.d. | 2009 | [129] |
| Orodispersible film | oral | 25, 50, 75, 100 | Maltodextrin, Glycerol, Polysorbate 20, Propylene glycol monocaprylate, Polyvinyl acetate dispersion 30%, Lemon and Grapefruit flavors (Lemon essential oil, Citral, Linalool, Grapefruit essential oil, Orange essential oil, Nootkaton, Butylated hydroxyanisol E320, Ascorbic acid E300, Maltodextrin, Arabic gum E414), Sucralose, Titanium dioxide, Indigotine | ED | Sildenafil Sandoz ODF | IBSA | 2013 | [130] |
| Chewable tablets | oral | 25, 50, 100 | Polacrilin potassium, silica colloidal anhydrous, lactose monohydrate, povidone K-30, aspartame (E951), croscarmellose sodium, peppermint flavor, magnesium stearate, potassium hydroxide (for pH adjustment) or hydrochloric acid (for pH adjustment). | ED | Sildenafil Portfarma | Portfarma | 2012 | [131] |
| Solution for injection | IV | 0.8 mg/mL | Glucose Water for injections | PAH | Revatio | Upjohn EESV | 2005 | [132] |
| Powder for suspension (after reconstitution) | oral | 10 mg/mL | Powder for oral suspension: Sorbitol Citric acid anhydrous, Sucralose, Sodium citrate, Xanthan gum, Titanium dioxide (E171), Sodium benzoate (E211), Silica, colloidal anhydrous Grape flavor: Maltodextrin, Grape juice concentrate, Gum acacia, Pineapple juice concentrate, Citric acid anhydrous, Natural flavoring | PAH | Revatio | Upjohn EESV | 2005 | [132] |
| Stationary Phase | Mobile Phase | Flow Rate/Temp | LOD (ng mL−1) | UV (nm) | Ref |
|---|---|---|---|---|---|
| Monolithic column Chromolith® RP-18e, (100 × 4.6 mm I.D) | Acetonitrile/water, 60/40, v/v | 2 mL min−1/Ambient | 25 | 292 | [152] |
| RP C18 | Phosphate buffer 10 mM (pH 6.5)/MeOH, gradient | 1 mL min−1/40 °C | 1.49 | 286 | [154] |
| Bondapak C18 (300 × 3.9 mm, 10 μm) | CH3COONH4 0.2 M (pH 7.0)/CH3CN, 50/50 v/v | 1 mL min−1/Ambient | 0.413 | 240 | [153] |
| Spherisorb® silica-C18 (250 × 4.6 mm, 5 μm) | TEA 0.2% v/v (pH = 3) with OPA and ACN (60/40 v/v) | 1 mL min−1/Ambient | 0.3 | 230 | [155] |
| Inertsil C18 (150 × 4.6 mm, 5 μm) | CH3CN/phosphate buffer (70/30 v/v, pH 7.0) | 0.8 mL min−1/Ambient | 1.8 | 228 | [156] |
| Inertsil®ODS-3; (250 × 4.6 mm, 3 μm) | CH3COONH4 0.2M (pH 7.0)/CH3CN, 50/50 v/v | 1.0 mL min−1/25 °C | 3.82 | 245 | [157] |
| Poroshell 120 EC-C18 (150 × 4.6 mm, 4 μm) | CH3COONH4 0.03M/CH3CN gradient | 1.0 mL min−1/40 °C | 0.67 | 230 | [158] |
| Analyte LC-MS | Sample | Sample Preparation | Analytical Parameters | Detection | LOD/LOQ | Ref |
|---|---|---|---|---|---|---|
| Sildenafil, N-desmethyl sildenafil | Human plasma | Protein precipitation using acetonitrile | Gradient elution using water and acetonitrile both containing 0.1% v/v formic acid Column: Gemini NX-C18 (50 × 4.6 mm i.d., 5 μm) + Gemini C18 guard column (4 × 3 mm i.d., 5 μm) Flow rate: 0.3 mL min−1 Temperature: 35 °C | MS/MS (MRM 1) | NM 2/2 ng mL−1 | [164] |
| Sildenafil, tadalafil, bosentan, ambrisentan, macitentan | Human plasma | (1) Protein precipitation using acetonitrile (2) SPE 3 (Oasis® HLB 96 well-plate) | Isocratic elution: 5 mM CH3COONH4/acetonitrile, 50/50 v/v Column: Symmetry C18 (150 × 2.1 mm i.d., 5 μm) Flow rate: 0.3 mL min−1 Temperature: 40 °C | MS | NM/1 ng mL−1 | [165] |
| Sildenafil, tadalafil, avanafil, vardenafil | Human plasma, urine | Magnetic SPE using citric acid coated iron oxide nanoparticles | Gradient elution using 10 mM HCOONH4 (pH 4.6) and acetonitrile containing 0.1% formic acid Column: Agilent Poroshell 120 EC-C18 (150 × 3.0 mm i.d., 2.7 μm) Flow rate: 0.55 mL min−1 Temperature: NM | QTOF-MS/MS | 0.74/2.45 ng g−1 | [166,167] |
| Sildenafil, tadalafil, vardenafil and avanafil | Human plasma, urine | Magnetic SPE using citric acid coated iron oxide nanoparticles | Gradient elution using 10 mM HCOONH4 (pH 4.6) and acetonitrile containing 0.1% formic acid Column: Agilent Poroshell 120 EC-C18 (150 × 3.0 mm i.d., 2.7 μm) + Agilent Zorbax Eclipse guard column (12.5 × 2.1 mm, 5 μm) Flow rate: 0.55 mL min−1 Temperature: NM | QTOF-MS/MS | 0.14/0.47 ng g−1 | [168] |
| Sildenafil, tadalafil, vardenafil and avanafil | Human urine, simulated gastric fluid | Dilution | Gradient elution using 10 mM HCOONH4 (pH 4.6) and acetonitrile containing 0.1% formic acid Column: Agilent Poroshell 120 EC-C18 (150 × 3.0 mm i.d., 2.7 μm) + Agilent Zorbax Eclipse guard column (12.5 × 2.1 mm, 5 μm) Flow rate: 0.55 mL min−1 Temperature: 40 °C | QTOF-MS/MS | 2.19/7.28 ng g−1 | [170,171,172,173] |
| Sildenafil, rosiglitazone | Rat plasma | Protein precipitation using methanol | Gradient elution using water and methanol both containing 0.1% formic acid Column: Kinetex C18 (50 × 2.1 mm i.d., 1.3 μm) Flow rate: 0.25 mL min−1 Temperature: 40 °C | MS/MS (MRM) | NM/5 ng mL−1 | [174,175,176] |
| Sildenafil, N-desmethyl sildenafil | Human plasma | Protein precipitation using acetonitrile | Gradient elution using water and acetonitrile both containing 0.05% formic acid Column: Acquity UPLC® HSS T3 C18 (150 × 2.1 mm i.d., 1.8 μm) Flow rate: 0.4 mL min−1 Temperature: 40 °C | MS/MS (MRM) | 1.95/3.9 ng mL | [30] |
| Sildenafil, mirodenafil, tadalafil, udenafil, vardenafil and their metabolites | Animal hair | Digestion with 5M HCl methanolic solution followed by mixed-mode SPE using C18 and strong ion exchange polymeric sorbents | Gradient elution using water and acetonitrile containing 0.1% formic acid Column: Agilent Poroshell 120 EC-C18 (50 × 3.0 mm i.d., 2.7 μm) Flow rate: 0.3 mL min−1 Temperature: 30 °C | MS/MS (MRM) | 0.05/0.1 ng mg−1 | [177,178,179] |
| Sosentan, ambrisentan, sildenafil, tadalafil | Human plasma | SPE using Oasis WAX cartridges | Isocratic elution using 5 mM CH3COONH4 (pH 5.0)/acetonitrile, 55/45 v/v Column: Cadenza CD-C18 (75 × 2.0 mm i.d., 3 μm) + Phenomenex Security guard column Flow rate: 0.2 mL min−1 Temperature: 40 °C | MS/MS (MRM) | NM/2 ng mL−1 | [180] |
| Sildenafil | Human plasma | Protein precipitation using acetonitrile followed by heating at 60 °C | Gradient elution using 50 mM CH3COONH4/3% trifluoroacetic acid/methanol/acetonitrile, 68/2/15/15 v/v/v/v Column: Agilent Poroshell 120 EC-C18 (50 × 3 mm i.d., 2.7 μm) Flow rate: 0.3 mL min−1 Temperature: 30 °C | MS/MS (MRM) | 7.25/10 ng mL−1 | [181] |
| Sildenafil | Dried blood spot | LLE 4 using diethyl ether | Isocratic elution using 2 mM CH3COONH4 (pH 5.0)/acetonitrile, 65/35 v/v Column: BEH C18 (50 × 2.1 mm i.d., 1.7 μm) Flow rate: 0.3 mL min−1 Temperature: 40 °C | MS/MS (MRM) | NM/5 ng mL−1 | [182] |
| Sildenafil, N-desmethyl sildenafil | Human plasma | LLE using methyl terb-butyl ether | Isocratic elution using 0.02% formic acid/acetonitrile, 30/70 v/v Column: Thermo Hypersil Gold (50 × 2.1 mm i.d., 5 μm) Flow rate: 0.5 mL min−1 Temperature: 35 °C | MS/MS (MRM) | NM | [183] |
| Sildenafil | Human plasma | SPE using Sep-Pak tC18 | Isocratic elution using 5 mM ammonium formate/acetonitrile, 60/40 v/v Column: Thermo Hypersil Gold (50 × 2.1 mm i.d., 5 μm) Flow rate: 0.5 mL min−1 Temperature: 35 °C | MS | NM/5 ng mL−1 | [184] |
| HPLC-UV | ||||||
| Sildenafil, avanafil, apomorphine, trazodone, yohimbine, tramadol, dapoxetine | Human plasma | Protein precipitation using acetonitrile | Gradient elution using sodium octanesulfonate, EDTA aqueous solution (pH 3.0) and acetonitrile or ethanol Column I: Chromolith Performance RP-18e (100 × 4.6 mm i.d. Column II: Poroshell core-shell EC-C18 (150 × 4.6 mm i.d., 2.7 μm) Flow rate: 1 or 2 mL min−1 Temperature: 35 °C | UV@210nm | 200/500 ng mL−1 (using Column I) 200/500 ng mL−1 (using Column II) | [167] |
| Sildenafil, tramadol | Rabbit plasma | SPE using Oasis HLB | Isocratic elution using 10 mM phosphate buffer (pH 7.5)/acetonitrile, 55/45 v/v Column: ODS Discovery HS C18 (150 × 4.6 mm i.d. 5 μm) Flow rate: 0.8 mL min−1 Temperature: Ambient | UV@220nm | 0.01/0.03 μg mL−1 | [169] |
| Sildenafil | Rat plasma | Protein precipitation | Isocratic elution using a mixture of acetonitrile and water (57.5/42.5 v/v) containing 0.675 mL trimethylamine (pH 7 with H3PO4) Column: Sepax Gp-C18 (150 × 4.6 mm i.d. 5 μm) Flow rate: 1 mL min−1 Temperature: 30 °C | UV@230nm | NM/20 ng mL−1 | [171] |
| Sildenafil, N-desmethyl sildenafil | Human plasma | LLE using ethyl acetate | Isocratic elution using a mixture of 30 mM phosphate buffer (pH 6.0)/acetonitrile, 53/47 v/v Column: Inertsil ODS2 C18 (150 × 4.6 mm i.d. 5 μm) Flow rate: 1 mL min−1 Temperature: 25 °C | UV@230nm | 0.5/1 ng mL−1 | [172] |
| Sildenafil | Human plasma | LLE using diethylacetate followed by back-extraction with 5% HClO4 aqueous solution | Isocratic elution using a mixture of water and acetonitrile (63/37 v/v) containing 0.1% TEA (pH 7.7) Column: Hypersil BDS-C18 (150 × 4.6 mm i.d. 5 μm) Flow rate: 1 mL min−1 Temperature: 25 °C | UV@230nm | NM/2 ng mL−1 | [175] |
| Sildenafil | Rat plasma | LLE using ethyl acetate/hexane (30/70 v/v) followed by back-extraction with mixture of methanol/0.1 M H2SO4 aqueous solution (10/90 v/v) | Isocratic elution using a mixture of 50 mM KH2PO4 (pH 4.5) and acetonitrile, 75/25 v/v Column: Supelcosil PCN cyanopropyl (250 × 4.6 mm i.d. 5 μm) Flow rate: 1 mL min−1 Temperature: 22 °C | UV@230nm | 5/10 ng mL−1 | [176] |
| aliskiren, prasugrel, rivaroxaban, rednisolone, propranolol, ketoprofen, nifedipine, naproxen, terbinafine, ibuprofen, diclofenac, sildenafil, acenocoumarol | Human urine | SPE using 17 different silica- and polymeric-based sorbents | Gradient elution using water and acetonitrile both containing 0.05% trifluoroacetic acid Column: Hypersil Gold C18 (50 × 2.1 mm i.d., 1.9 μm) Flow rate: 0.5–1.0 mL min−1 Temperature: 25 °C | UV@228nm | 66/198 ng mL−1 | [179] |
| Sildenafil, N-desmethyl sildenafil | Human plasma | LLE using ethyl acetate | Isocratic elution using a mixture of 30 mM KH2PO4 (pH 4.5) and acetonitrile, 53/47 v/v Column: μBondapack C18 (150 × 3.9 mm i.d. 5 μm) Flow rate: 0.8 mL min−1 Temperature: 21 °C | UV@230nm | 1/10 ng mL−1 | [185] |
| Sildenafil, vardenafil, aildenafil | Human plasma | Ionic liquid-based dispersive liquid liquid microextraction followed by back-extraction with 10% acetic acid | Isocratic elution using a mixture of water and methanol both containing 1% acetic acid, 60/40 v/v Column: Shimazdzu RP-C18 (250 × 4.6 mm i.d. 5 μm) Flow rate: 1.2 mL min−1 Temperature: NM | UV@254nm | 0.17/NM | [186] |
| Voltammetry | ||||||
| Sildenafil | Human serum | Dilution | Screen-printed electrode with carbon working and auxiliary electrodes and silver reference electrode Potential range: −0.75 to 1.55 V Scan rate: 175 mV s−1 Sample medium: 0.15 M acetate buffer (pH 5.0) | − | 5.9 × 10−10/2.0 × 10−9 mol L−1 | [151] |
| Sildenafil | Human serum | NM | Square wave voltammetry using polycrystalline gold (surface area 0.5 cm2), gold wire and saturated calomel electrode as working, counter and reference electrodes respectively Sample medium: 0.05 M NaHCO3 | − | 0.031/0.106 μmol L−1 | [173] |
| Sildenafil | Simulated human urine | Dilution | Cyclic voltammetry using screen-printed glassy carbon electrode modified gold nanoparticles via electrodeposition Sample medium: Britton-Robinson buffer (pH 7.3) | − | 5.2 × 10−10 mol L−1/NM | [178] |
| Batch Spectrophotometry | ||||||
| Sildenafil | Human urine | Dispersive solid-phase microextraction using Mn@ CuS/ZnS nanocomposite loaded on activated carbon | − | NM | 2.5/8.35 ng mL−1 | [181] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouranidis, A.; Tsiaxerli, A.; Vardaka, E.; Markopoulou, C.K.; Zacharis, C.K.; Nicolaou, I.; Hatzichristou, D.; Haidich, A.-B.; Kostomitsopoulos, N.; Kachrimanis, K. Sildenafil 4.0—Integrated Synthetic Chemistry, Formulation and Analytical Strategies Effecting Immense Therapeutic and Societal Impact in the Fourth Industrial Era. Pharmaceuticals 2021, 14, 365. https://doi.org/10.3390/ph14040365
Ouranidis A, Tsiaxerli A, Vardaka E, Markopoulou CK, Zacharis CK, Nicolaou I, Hatzichristou D, Haidich A-B, Kostomitsopoulos N, Kachrimanis K. Sildenafil 4.0—Integrated Synthetic Chemistry, Formulation and Analytical Strategies Effecting Immense Therapeutic and Societal Impact in the Fourth Industrial Era. Pharmaceuticals. 2021; 14(4):365. https://doi.org/10.3390/ph14040365
Chicago/Turabian StyleOuranidis, Andreas, Anastasia Tsiaxerli, Elisavet Vardaka, Catherine K. Markopoulou, Constantinos K. Zacharis, Ioannis Nicolaou, Dimitris Hatzichristou, Anna-Bettina Haidich, Nikolaos Kostomitsopoulos, and Kyriakos Kachrimanis. 2021. "Sildenafil 4.0—Integrated Synthetic Chemistry, Formulation and Analytical Strategies Effecting Immense Therapeutic and Societal Impact in the Fourth Industrial Era" Pharmaceuticals 14, no. 4: 365. https://doi.org/10.3390/ph14040365