Fermentation Strategies for Production of Pharmaceutical Terpenoids in Engineered Yeast
Abstract
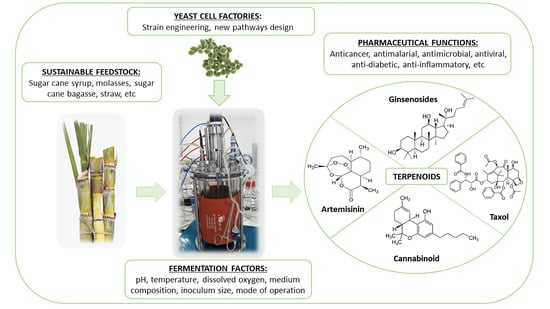
:1. Introduction
2. Pharmaceutical Terpenoids
2.1. Artemisinin and Its Derivatives
2.2. Paclitaxel (Taxol)
2.3. Cannabinoids
2.4. Other Medically Important Terpenoids
3. Biosynthesis of Medically Important Terpenoids
4. Metabolic Engineering Strategies for Pharmaceutic Terpenoids Production in Yeast
- Modifying endogenous pathways for synthesis of desired terpenoids.
- Finding and introducing new heterologous enzymes and pathways into yeast.
- Determination of rate limiting steps in selected pathways by application of omics studies.
- Elimination of rate limiting steps in target pathways via overexpression of genes, and cofactor and transporter engineering.
- Developing enzyme activity and/or specificity by protein engineering.
- Improving expression level of target key enzymes.
- Blocking or down regulating competing pathways.
- Increasing precursor and cofactor supply.
- Balancing cell growth and terpenoid synthesis for fermentation process.
5. Factors Affecting Fermentation Process of Pharmaceutical Terpenoids
5.1. Strain Engineering
5.2. Carbon Source
5.3. Nitrogen Source
5.4. pH
5.5. Temperature
5.6. Dissolved Oxygen
5.7. Inoculum Size
6. Modes of Fermentation Process
6.1. Batch Mode
6.2. Fed-Batch Mode
6.3. Continuous Mode
7. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christianson, D.W. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belcher, M.S.; Mahinthakumar, J.; Keasling, J.D. New frontiers: Harnessing pivotal advances in microbial engineering for the biosynthesis of plant-derived terpenoids. Curr. Opin. Biotechnol. 2020, 65, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, M.; Zhao, G.-R.; Lu, W. Harnessing Yeast Peroxisomes and Cytosol Acetyl-CoA for Sesquiterpene α-Humulene Production. J. Agric. Food Chem. 2020, 68, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Moser, S.; Pichler, H. Identifying and engineering the ideal microbial terpenoid production host. Appl. Microbiol. Biotechnol. 2019, 103, 5501–5516. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Quan, S.; Xiao, H. Towards efficient terpenoid biosynthesis: Manipulating IPP and DMAPP supply. Bioresour. Bioprocess. 2019, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Nielsen, J.; Liu, Z. Engineering yeast metabolism for production of terpenoids for use as perfume ingredients, pharmaceuticals and biofuels. FEMS Yeast Res. 2017, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.H.; Thulasingam, S.; Nagarajan, S. Terpenoids as anti-colon cancer agents—A comprehensive review on its mechanistic perspectives. Eur. J. Pharmacol. 2017, 795, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Krivoruchko, A.; Nielsen, J. Production of natural products through metabolic engineering of Saccharomyces cerevisiae. Curr. Opin. Biotechnol. 2015, 35, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Leavell, M.D.; McPhee, D.J.; Paddon, C.J. Developing fermentative terpenoid production for commercial usage. Curr. Opin. Biotechnol. 2016, 37, 114–119. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Y.; Wang, M. Discovery and Engineering of Cytochrome P450s for Terpenoid Biosynthesis. Trends Biotechnol. 2019, 37, 618–631. [Google Scholar] [CrossRef]
- Benjamin, K.R.; Silva, I.R.; Cherubim, J.P.; McPhee, D.; Paddon, C.J. Developing Commercial Production of Semi-Synthetic Artemisinin, and of β-Farnesene, an Isoprenoid Produced by Fermentation of Brazilian Sugar. J. Braz. Chem. Soc. 2016, 27, 1339–1345. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biggs, B.W.; Lim, C.G.; Saglinani, K.; Shankar, S.; Stephanopoulos, G.; De Mey, M.; Ajikumar, P.K. Overcoming heterologous protein interdependency to optimize P450-mediated Taxol precursor synthesis in Escherichia coli. Proc. Natl. Acad. Sci. USA 2016, 113, 3209–3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandy, S.K.; Srivastava, R. A review on sustainable yeast biotechnological processes and applications. Microbiol. Res. 2018, 207, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Alexander, W.G. A history of genome editing inSaccharomyces cerevisiae. Yeast 2017, 35, 355–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Z.; Zhang, L.; Zhu, S.; Yuan, W.; Hang, J.; Yin, D.; Tang, X.; Zheng, J.; Wang, Z.; Sun, J. Overexpression of the transcription factor HAC1 improves nerolidol production in engineered yeast. Enzym. Microb. Technol. 2020, 134, 109485. [Google Scholar] [CrossRef]
- Hansen, N.L.; Miettinen, K.; Zhao, Y.; Ignea, C.; Andreadelli, A.; Raadam, M.H.; Makris, A.M.; Møller, B.L.; Stærk, D.; Bak, S.; et al. Integrating pathway elucidation with yeast engineering to produce polpunonic acid the precursor of the anti-obesity agent celastrol. Microb. Cell Factories 2020, 19, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, X.; Cha, Y.; Li, W.; Zhu, C.; Zhu, M.; Li, S.; Zhuo, M.; Huang, S.; Li, J. Stepwise engineering of Saccharomyces cerevisiae to produce (+)-valencene and its related sesquiterpenes. RSC Adv. 2019, 9, 30171–30181. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Liu, M.; Li, Z.-H.; Tao, X.; Wei, D.-Z.; Wang, F.-Q. Significantly Enhanced Production of Patchoulol in Metabolically Engineered Saccharomyces cerevisiae. J. Agric. Food Chem. 2019, 67, 8590–8598. [Google Scholar] [CrossRef]
- Wang, C.; Liwei, M.; Park, J.-B.; Jeong, S.-H.; Wei, G.; Wang, Y.; Kim, S.-W. Microbial platform for terpenoid production: Escherichia coli and Yeast. Front. Microbiol. 2018, 9, 2460. [Google Scholar] [CrossRef]
- Zhuang, X.; Kilian, O.; Monroe, E.; Ito, M.; Tran-Gymfi, M.B.; Liu, F.; Davis, R.W.; Mirsiaghi, M.; Sundstrom, E.; Pray, T.; et al. Monoterpene production by the carotenogenic yeast Rhodosporidium toruloides. Microb. Cell Factories 2019, 18, 1–15. [Google Scholar] [CrossRef]
- Lin, P.-C.; Pakrasi, H.B. Engineering cyanobacteria for production of terpenoids. Planta 2019, 249, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nat. Cell Biol. 2016, 537, 694–697. [Google Scholar] [CrossRef]
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.J.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nat. Cell Biol. 2013, 496, 528–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, P.; Benjamin, K.; Bhattacharjee, B.; Garcia, F.; Leng, J.; Liu, C.-L.; Murarka, A.; Pitera, D.; Porcel, E.M.R.; Da Silva, I.; et al. Clean manufacturing powered by biology: How Amyris has deployed technology and aims to do it better. J. Ind. Microbiol. Biotechnol. 2020, 47, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Ateba, S.B.; Mvondo, M.A.; Ngeu, S.T.; Tchoumtchoua, J.; Awounfack, C.F.; Njamen, D.; Krenn, L. Natural Terpenoids Against Female Breast Cancer: A 5-year Recent Research. Curr. Med. Chem. 2018, 25, 3162–3213. [Google Scholar] [CrossRef]
- Jaeger, R.; Cuny, E. Terpenoids with Special Pharmacological Significance: A Review. Nat. Prod. Commun. 2016, 11, 1373–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manayi, A.; Nabavi, S.M.; Daglia, M.; Jafari, S. Natural terpenoids as a promising source for modulation of GABAergic system and treatment of neurological diseases. Pharmacol. Rep. 2016, 68, 671–679. [Google Scholar] [CrossRef]
- Xu, Y.F.; Lian, D.W.; Chen, Y.Q.; Cai, Y.F.; Zheng, Y.F.; Fan, P.L.; Ren, W.K.; Fu, L.J.; Li, Y.C.; Xie, J.H.; et al. In Vitro and In Vivo Antibacterial Activities of Patchouli Alcohol, a Naturally Occurring Tricyclic Sesquiterpene, against Helicobacter pylori Infection. Antimicrob. Agents Chemother. 2017, 61, e00122-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Xin, X.; Zhai, X.; Xia, Z.; Shen, K. Sequential combination of flavopiridol with Taxol synergistically suppresses human ovarian carcinoma growth. Arch. Gynecol. Obstet. 2014, 291, 143–150. [Google Scholar] [CrossRef]
- Carvalho, Â.; Hansen, E.H.; Kayser, O.; Carlsen, S.; Stehle, F. Designing microorganisms for heterologous biosynthesis of cannabinoids. FEMS Yeast Res. 2017, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wei, W.; Ye, W.; Li, X.; Zhao, W.; Yang, C.; Li, C.; Yan, X.; Zhou, Z. Synthesizing ginsenoside Rh2 in Saccharomyces cerevisiae cell factory at high-efficiency. Cell Discov. 2019, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yi, Y.-S.; Kim, M.-Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.-R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Zha, W.; Zi, J. Biotechnological production of betulinic acid and derivatives and their applications. Appl. Microbiol. Biotechnol. 2020, 104, 3339–3348. [Google Scholar] [CrossRef] [PubMed]
- Meshnick, S.R. Artemisinin: Mechanisms of action, resistance and toxicity. Int. J. Parasitol. 2002, 32, 1655–1660. [Google Scholar] [CrossRef]
- Tu, Y. ChemInform Abstract: Artemisinin—A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. Ed. 2016, 47, 10210–10226. [Google Scholar] [CrossRef]
- Creek, D.J.; Charman, W.N.; Chiu, F.C.K.; Prankerd, R.J.; Dong, Y.; Vennerstrom, J.L.; Charman, S.A. Relationship between Antimalarial Activity and Heme Alkylation for Spiro- and Dispiro-1,2,4-Trioxolane Antimalarials. Antimicrob. Agents Chemother. 2008, 52, 1291–1296. [Google Scholar] [CrossRef] [Green Version]
- Efferth, T.; Dunstan, H.; Sauerbrey, A.; Miyachi, H.; Chitambar, C. The anti-malarial artesunate is also active against cancer. Int. J. Oncol. 2001, 18, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Appalasamy, S.; Lo, K.Y.; Ch’Ng, S.J.; Nornadia, K.; Othman, A.S.; Chan, L.-K. Antimicrobial Activity of Artemisinin and Precursor Derived fromIn VitroPlantlets ofArtemisia annuaL. BioMed Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.-S.; Choi, W.J.; Lee, S.; Kim, W.J.; Lee, D.C.; Sohn, U.D.; Shin, H.-S.; Kim, W. Anti-inflammatory, Antioxidant and Antimicrobial Effects of Artemisinin Extracts fromArtemisia annuaL. Korean J. Physiol. Pharmacol. 2014, 19, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohlfarth, C.; Efferth, T. Natural products as promising drug candidates for the treatment of hepatitis B and C. Acta Pharmacol. Sin. 2008, 30, 25–30. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, E.-S.R.; Ahmed, A.S.; Hassan, I.A.; Ismaiel, A.A.; El-Din, A.-Z.A.K. Semi-continuous production of the anticancer drug taxol by Aspergillus fumigatus and Alternaria tenuissima immobilized in calcium alginate beads. Bioprocess. Biosyst. Eng. 2020, 43, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, R. Estrogenic terpenes and terpenoids: Pathways, functions and applications. Eur. J. Pharmacol. 2017, 815, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Syriac, A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J. Assist. Reprod. Genet. 2015, 32, 1575–1588. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2006, 147, S163–S171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Reiter, M.A.; D’Espaux, L.; Wong, J.; Denby, C.M.; Lechner, A.; Zhang, Y.; Grzybowski, A.T.; Harth, S.; Lin, W.; et al. Author Correction: Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nat. Cell Biol. 2020, 580, E2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajikumar, P.K.; Tyo, K.; Carlsen, S.; Mucha, O.; Phon, T.H.; Stephanopoulos, G. Terpenoids: Opportunities for Biosynthesis of Natural Product Drugs Using Engineered Microorganisms. Mol. Pharm. 2008, 5, 167–190. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; So, I.; Chun, J.N.; Jeon, J.-H. The antitumor effects of geraniol: Modulation of cancer hallmark pathways (Review). Int. J. Oncol. 2016, 48, 1772–1782. [Google Scholar] [CrossRef] [Green Version]
- Jongedijk, E.; Cankar, K.; Buchhaupt, M.; Schrader, J.; Bouwmeester, H.; Beekwilder, J. Biotechnological production of limonene in microorganisms. Appl. Microbiol. Biotechnol. 2016, 100, 2927–2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Song, K.; Zhang, Y.; Chu, C.; Fan, B.; Song, Y.; Huang, H.; Chen, G. Biotransformation of betulinic acid by Circinella muscae and Cunninghamella echinulata to discover anti-inflammatory derivatives. Phytochemistry 2021, 182, 112608. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Lee, J.; Chen, W.N. Potential Natural Food Preservatives and Their Sustainable Production in Yeast: Terpenoids and Polyphenols. J. Agric. Food Chem. 2019, 67, 4397–4417. [Google Scholar] [CrossRef] [PubMed]
- Paramasivan, K.; Mutturi, S. Progress in terpene synthesis strategies through engineering of Saccharomyces cerevisiae. Crit. Rev. Biotechnol. 2017, 37, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Deng, Z.; Liu, T. Strategies for terpenoid overproduction and new terpenoid discovery. Curr. Opin. Biotechnol. 2017, 48, 234–241. [Google Scholar] [CrossRef]
- Urlacher, V.B.; Girhard, M. Cytochrome P450 monooxygenases: An update on perspectives for synthetic application. Trends Biotechnol. 2012, 30, 26–36. [Google Scholar] [CrossRef]
- Westfall, P.J.; Pitera, D.J.; Lenihan, J.R.; Eng, D.; Woolard, F.X.; Regentin, R.; Horning, T.; Tsuruta, H.; Melis, D.J.; Owens, A.; et al. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc. Natl. Acad. Sci. USA 2012, 109, E111–E118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalcinati, G.; Partow, S.; Siewers, V.; Schalk, M.; Daviet, L.; Nielsen, J. Combined metabolic engineering of precursor and co-factor supply to increase α-santalene production by Saccharomyces cerevisiae. Microb. Cell Factories 2012, 11, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignea, C.; Pontini, M.; Motawia, M.S.; Maffei, M.E.; Makris, A.M.; Kampranis, S.C. Synthesis of 11-carbon terpenoids in yeast using protein and metabolic engineering. Nat. Chem. Biol. 2018, 14, 1090–1098. [Google Scholar] [CrossRef]
- Ignea, C.; Raadam, M.H.; Motawia, M.S.; Makris, A.M.; Vickers, C.E.; Kampranis, S.C. Orthogonal monoterpenoid biosynthesis in yeast constructed on an isomeric substrate. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Lu, W. Insight into yeast: A study model of lipid metabolism and terpenoid biosynthesis. Biotechnol. Appl. Biochem. 2014, 62, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, C.; Lindley, N.D. Metabolic Engineering Strategies for Sustainable Terpenoid Flavor and Fragrance Synthesis. J. Agric. Food Chem. 2020, 68, 10252–10264. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Ouellet, M.; Chan, R.; Mukhopadhyay, A.; Keasling, J.D.; Lee, T.S. Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun. 2011, 2, 483. [Google Scholar] [CrossRef] [Green Version]
- Albertsen, L.; Chen, Y.; Bach, L.S.; Rattleff, S.; Maury, J.; Brix, S.; Nielsen, J.; Mortensen, U.H. Diversion of Flux toward Sesquiterpene Production inSaccharomyces cerevisiaeby Fusion of Host and Heterologous Enzymes. Appl. Environ. Microbiol. 2010, 77, 1033–1040. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Sun, M.; Xu, S.; Zhou, J. Enhanced (S)-linalool production by fusion expression of farnesyl diphosphate synthase and linalool synthase in Saccharomyces cerevisiae. J. Appl. Microbiol. 2016, 121, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Li, C.; Zhang, Y.; Shen, Y.; Hou, J.; Bao, X. Dynamic control of ERG20 expression combined with minimized endogenous downstream metabolism contributes to the improvement of geraniol production in Saccharomyces cerevisiae. Microb. Cell Factories 2017, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ignea, C.; Pontini, M.; Maffei, M.E.; Makris, A.M.; Kampranis, S.C. Engineering Monoterpene Production in Yeast Using a Synthetic Dominant Negative Geranyl Diphosphate Synthase. ACS Synth. Biol. 2014, 3, 298–306. [Google Scholar] [CrossRef]
- Zhang, G.; Cao, Q.; Liu, J.; Liu, B.; Li, J.; Li, C. Refactoring β-amyrin synthesis inSaccharomyces cerevisiae. AIChE J. 2015, 61, 3172–3179. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, X.; Jiang, G.; Wu, J.; Zhang, J.-L.; Lei, D.; Yuan, Y.-J.; Qiao, J.; Zhao, G.-R. Orthogonal Engineering of Biosynthetic Pathway for Efficient Production of Limonene in Saccharomyces cerevisiae. ACS Synth. Biol. 2019, 8, 968–975. [Google Scholar] [CrossRef]
- Jiang, G.-Z.; Yao, M.-D.; Wang, Y.; Zhou, L.; Song, T.-Q.; Liu, H.; Xiao, W.-H.; Yuan, Y.-J. Manipulation of GES and ERG20 for geraniol overproduction in Saccharomyces cerevisiae. Metab. Eng. 2017, 41, 57–66. [Google Scholar] [CrossRef]
- Zhao, F.; Du, Y.; Bai, P.; Liu, J.; Lu, W.; Yuan, Y. Enhancing Saccharomyces cerevisiae reactive oxygen species and ethanol stress tolerance for high-level production of protopanoxadiol. Bioresour. Technol. 2017, 227, 308–316. [Google Scholar] [CrossRef]
- Czarnotta, E.; Dianat, M.; Korf, M.; Granica, F.; Merz, J.; Maury, J.; Jacobsen, S.A.B.; Förster, J.; Ebert, B.E.; Blank, L.M. Fermentation and purification strategies for the production of betulinic acid and its lupane-type precursors in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2017, 114, 2528–2538. [Google Scholar] [CrossRef] [Green Version]
- Özaydın, B.; Burd, H.; Lee, T.S.; Keasling, J.D. Carotenoid-based phenotypic screen of the yeast deletion collection reveals new genes with roles in isoprenoid production. Metab. Eng. 2013, 15, 174–183. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Zhao, F.; Lu, C.; Zhao, G.-R.; Lu, W. Production of sesquiterpenoid zerumbone from metabolic engineered Saccharomyces cerevisiae. Metab. Eng. 2018, 49, 28–35. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, Y.; Huang, L.; Zhang, X. Production of miltiradiene by metabolically engineeredSaccharomyces cerevisiae. Biotechnol. Bioeng. 2012, 109, 2845–2853. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, Y.; Zhang, X.; Shi, M.; Wang, B.; Wang, D.; Huang, L.; Zhang, X. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab. Eng. 2013, 20, 146–156. [Google Scholar] [CrossRef]
- Behrendorff, J.B.; E Vickers, C.; Chrysanthopoulos, P.; Nielsen, L.K. 2,2-Diphenyl-1-picrylhydrazyl as a screening tool for recombinant monoterpene biosynthesis. Microb. Cell Factories 2013, 12, 76. [Google Scholar] [CrossRef] [Green Version]
- Jongedijk, E.; Cankar, K.; Ranzijn, J.; Van Der Krol, S.; Bouwmeester, H.; Beekwilder, J. Capturing of the monoterpene olefin limonene produced inSaccharomyces cerevisiae. Yeast 2014, 32, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.; D’Espaux, L.; Dev, I.; van der Horst, C.; Keasling, J. De novo synthesis of the sedative valerenic acid in Saccharomyces cerevisiae. Metab. Eng. 2018, 47, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Šantek, M.I.; Komes, D.; Novak, S.; Šantek, B. Bioethanol Production from Renewable Raw Materials and its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Peng, B.; Plan, M.R.; Carpenter, A.; Nielsen, L.K.; Vickers, C.E. Coupling gene regulatory patterns to bioprocess conditions to optimize synthetic metabolic modules for improved sesquiterpene production in yeast. Biotechnol. Biofuels 2017, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.S.; Ikram, S.; Rasool, A.; Li, C. Design and construction of short synthetic terminators for β-amyrin production in Saccharomyces cerevisiae. Biochem. Eng. J. 2019, 146, 105–116. [Google Scholar] [CrossRef]
- Gruchattka, E.; Hädicke, O.; Klamt, S.; Schütz, V.; Kayser, O. In silico profiling of Escherichia coli and Saccharomyces cerevisiae as terpenoid factories. Microb. Cell Factories 2013, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- Cunha, J.T.; Soares, P.O.; Romaní, A.; Thevelein, J.M.; Domingues, L. Xylose fermentation efficiency of industrial Saccharomyces cerevisiae yeast with separate or combined xylose reductase/xylitol dehydrogenase and xylose isomerase pathways. Biotechnol. Biofuels 2019, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.C.; Meyer, S.; Claudel, P.; Bergdoll, M.; Karst, F. Metabolic engineering of monoterpene synthesis in yeast. Biotechnol. Bioeng. 2011, 108, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Tokuhiro, K.; Muramatsu, M.; Ohto, C.; Kawaguchi, T.; Obata, S.; Muramoto, N.; Hirai, M.; Takahashi, H.; Kondo, A.; Sakuradani, E.; et al. Overproduction of Geranylgeraniol by Metabolically Engineered Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009, 75, 5536–5543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, K.; Qiao, K.; Edgar, S.M.; Stephanopoulos, G. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat. Biotechnol. 2015, 33, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhao, X.; Zou, H.; Fu, J.; Du, G.; Zhou, J.; Chen, J. Comparative proteomic analysis of Saccharomyces cerevisiae under different nitrogen sources. J. Proteom. 2014, 101, 102–112. [Google Scholar] [CrossRef]
- Ter Schure, E. The role of ammonia metabolism in nitrogen catabolite repression in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2000, 24, 67–83. [Google Scholar] [CrossRef] [Green Version]
- Torija, M.J.; Beltran, G.; Novo, M.; Poblet, M.; Rozès, N.; Guillamón, J.M.; Mas, A. Effect of the nitrogen source on the fatty acid composition of Saccharomyces cerevisiae. Food Microbiol. 2003, 20, 255–258. [Google Scholar] [CrossRef]
- Shang, F.; Wen, S.; Wang, X.; Tan, T. Effect of nitrogen limitation on the ergosterol production by fed-batch culture of Saccharomyces cerevisiae. J. Biotechnol. 2006, 122, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, C.M.; Ayson, M.; Moss, N.; Lieu, B.; Jackson, P.; Gaucher, S.P.; Horning, T.; Dahl, R.H.; Denery, J.R.; Abbott, D.A.; et al. Use of pantothenate as a metabolic switch increases the genetic stability of farnesene producing Saccharomyces cerevisiae. Metab. Eng. 2014, 25, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moreno, R.; Morales, P.; Gonzalez, R.; Mas, A.; Beltran, G. Biomass production and alcoholic fermentation performance of Saccharomyces cerevisiae as a function of nitrogen source. FEMS Yeast Res. 2012, 12, 477–485. [Google Scholar] [CrossRef]
- Vendramini, C.; Beltran, G.; Nadai, C.; Giacomini, A.; Mas, A.; Corich, V. The role of nitrogen uptake on the competition ability of three vineyard Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2017, 258, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narendranath, N.V.; Power, R. Relationship between pH and Medium Dissolved Solids in Terms of Growth and Metabolism of Lactobacilli and Saccharomyces cerevisiae during Ethanol Production. Appl. Environ. Microbiol. 2005, 71, 2239–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants—Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muramatsu, M.; Ohto, C.; Obata, S.; Sakuradani, E.; Shimizu, S. Alkaline pH enhances farnesol production by Saccharomyces cerevisiae. J. Biosci. Bioeng. 2009, 108, 52–55. [Google Scholar] [CrossRef]
- Chen, H.; Li, M.; Liu, C.; Zhang, H.; Xian, M.; Liu, H. Enhancement of the catalytic activity of Isopentenyl diphosphate isomerase (IDI) from Saccharomyces cerevisiae through random and site-directed mutagenesis. Microb. Cell Factories 2018, 17, 1–14. [Google Scholar] [CrossRef]
- Torija, M.J. Effects of fermentation temperature on the strain population of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2003, 80, 47–53. [Google Scholar] [CrossRef]
- Zabed, H.; Faruq, G.; Sahu, J.N.; Azirun, M.S.; Hashim, R.; Boyce, A.N. Bioethanol Production from Fermentable Sugar Juice. Sci. World J. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, T.Q.; Santos, S.B.; Martins, I.M.; Domingues, L.; Oliveira, C. Production and Bioengineering of Recombinant Pharmaceuticals. In Proteins: Sustainable Source, Processing and Applications; Elsevier: London, UK, 2019; pp. 259–293. [Google Scholar] [CrossRef]
- Oliveira, C.; Teixeira, J.A.; Domingues, L. Recombinant production of plant lectins in microbial systems for biomedical application—The frutalin case study. Front. Plant. Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, T.; Zhang, M.; Gao, H. Ergosterol production by fed-batch fermentation of Saccharomyces cerevisiae. Enzym. Microb. Technol. 2003, 33, 366–370. [Google Scholar] [CrossRef]
- Belo, I.; Pinheiro, R.; Mota, M. Morphological and physiological changes in Saccharomyces cerevisiae by oxidative stress from hyperbaric air. J. Biotechnol. 2005, 115, 397–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesquita, T.J.B.; Sargo, C.R.; Fuzer, J.R.; Paredes, S.A.H.; Giordano, R.D.C.; Horta, A.C.L.; Zangirolami, T.C. Metabolic fluxes-oriented control of bioreactors: A novel approach to tune micro-aeration and substrate feeding in fermentations. Microb. Cell Factories 2019, 18, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Zhang, G.; Qin, L.; Li, J.; Li, C. Simultaneously down-regulation of multiplex branch pathways using CRISPRi and fermentation optimization for enhancing β-amyrin production in Saccharomyces cerevisiae. Synth. Syst. Biotechnol. 2019, 4, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Mantzouridou, F.; Naziri, E.; Tsimidou, M.Z. Squalene versus Ergosterol Formation Using Saccharomyces cerevisiae: Combined Effect of Oxygen Supply, Inoculum Size, and Fermentation Time on Yield and Selectivity of the Bioprocess. J. Agric. Food Chem. 2009, 57, 6189–6198. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.-Z.; Tian, H.-C.; Cheng, J.-S.; Yuan, Y.-J. Inoculum size-dependent interactive regulation of metabolism and stress response of Saccharomyces cerevisiae revealed by comparative metabolomics. J. Biotechnol. 2009, 144, 279–286. [Google Scholar] [CrossRef]
- Sood, S.; Singhal, R.; Bhat, S.; Kumar, A. Inoculum Preparation. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 151–164. [Google Scholar]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Culture preservation and inoculum development. In Principles of Fermentation Technology; Elsevier: Oxford, UK, 2017; pp. 335–399. [Google Scholar]
- Subramaniam, R. High-density Cultivation in the Production of Microbial Products. Chem. Biochem. Eng. Q. 2019, 32, 451–464. [Google Scholar] [CrossRef]
- Song, C.W.; Park, J.M.; Chung, S.C.; Lee, S.Y.; Song, H. Microbial production of 2,3-butanediol for industrial applications. J. Ind. Microbiol. Biotechnol. 2019, 46, 1583–1601. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Rohany, S.; Safaiyan, S. Biotransformation of citral by free and immobilized Saccharomyces cerevisiae. Chem. Nat. Compd. 2012, 48, 322–324. [Google Scholar] [CrossRef]
- Khor, G.K.; Uzir, M.H. Saccharomyces cerevisiae: A potential stereospecific reduction tool for biotransformation of mono- and sesquiterpenoids. Yeast 2010, 28, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Saarela, U.; Leiviskä, K.; Juuso, E. Modelling of a fed-batch fermentation process. Most 2003, 21, 1–23. [Google Scholar]
- Gunther, J.; Seborg, D.; Baclaski, J. Fault detection and diagnosis in industrial fed-batch fermentation. In Proceedings of the 2006 American Control Conference, Minneapolis, MN, USA, 14–16 June 2006; Volume 2006. [Google Scholar] [CrossRef]
- Ebert, B.E.; Czarnotta, E.; Blank, L.M. Physiologic and metabolic characterization of Saccharomyces cerevisiae reveals limitations in the synthesis of the triterpene squalene. FEMS Yeast Res. 2018, 18, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Classification of Terpene | Terpene Name | Pharmaceutical Function | References |
|---|---|---|---|
| Monoterpene | Perillyl alcohol | Anticancer | [3] |
| Geraniol | Anticancer | ||
| D-limonene | Anticancer, transdermal absorption of drugs | ||
| Menthol | Antimicrobial, transdermal absorption of drugs | ||
| Sabinene | Antimicrobial | ||
| Sesquiterpene | Artemisinin and its derivatives | Antimalarial, anticancer, antibacterial, antiviral activities and hypoglycemic effect | [3,32] |
| Patchoulol | Antibacterial activity | ||
| Diterpene | Paclitaxel | Anti-ovarian, breast, colorectal, head and neck cancers, small-cell and non-small-cell lung cancers (NSCLCs), and treatment of AIDS | [3,8,14,33] |
| Meroterpene | Cannabinoids | Treatment of pain relieving conditions (in cancer chemotherapy, AIDS, and multiple sclerosis) | [8,34] |
| Triterpene | Ginsenosides | Anti-oxidation, anti-inflammatory, hepatoprotection, anti-diabetic (hypoglycemic activity) and anti-tumor | [3,35,36] |
| Betulinic acid and its derivatives | Anticancer, anti-inflammatory, anti-diabetic, antimicrobial and anti-human immunodeficiency virus (HIV) | [37,38] |
| Compound | Titer | Strategy | References |
|---|---|---|---|
| Amorpha-4,11-diene | >40 g/L | Overexpression of ADS, upc2-1 Integration of genes copies using control of galactose-inducible promoters: 3 copies of tHMG1, and ERG10, 13, 12, 8, and IDI1 Deletion of gal80Δ Downregulation of ERG9 | [60] |
| Artemisinic acid | 25 g/L | Overexpression of ADS, CYP71AV1, CPR1, CYB5, ALDH1 and ADH1 from A. annua, HEM1, and CTT1 under control of galactose-inducible promoters Deletion of gal80Δ Downregulation of ERG9 | [27] |
| Farnesene | 130 g/L | Overexpression of ADA, xPK, PTA, NADH-HMGr, Farnesene synthase Deletion of adh1 Δ, ald4 Δ, ald6Δ, gpp1Δ, gal2Δ, bdh1Δ Overexpression of enzymes of the MVA pathway to Erg20 Downregulation of ERG9 | [26] |
| Bisabolone | >900 mg/L | Overexpression of tHMG1, ERG20, upc2-1 and bisabolene synthase Downregulation of ERG9 | [66] |
| Alpha-Santalene | 92 mg/L | Overexpression of tHMG1 Deletion of Ipp1Δ, dpp1Δ Downregulation of ERG9 | [61] |
| Patchoulol | 42.1 mg/L | Overexpression of ERG20 and PatTps177 Downregulation of ERG9 | [67] |
| (S)-Linalool | 0.26 mg/L | Overexpression of Erg20 and (S)-linalool synthase Diploid | [68] |
| Geraniol | 1.69 g/L | 2μ plasmid of PTEF1-tVoGES-(GGGS)-ERG20WW fusion protein 2μ plasmid of PTEF1-tHMG1, PPGK1-IDI1, PTEF1- upc2.1 PHXT1-ERG20, oye2Δ | [69] |
| Sabinene | 1.75 mg/L | 2μ plasmid of PTDH3-ERG20 (F96W-N127W)- sabinene synthase (Salvia pomifera) fusion protein Diploid ERG9/erg9, ERG20/erg20, PGal1-HMG2 (K6R), PTDH3-HMG2 (K6R) × 2 | [70] |
| Group | Product | Titer (mg/L) | Carbon Source | Nitrogen Source | pH | °C | Aeration /Agitation | Dissolved Oxygen | Inoculum Size | Feeding Strategy | Operation Mode | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoterpenes | Limonene | 1.48 | 2 g/L glucose and 18 g/L galactose | (NH4)2SO4 | - | 30 | 200 rpm | - | 1 OD = 0.05 | - | Shake-flask | [80] |
| (-)-Limonene | 0.49 | 20 g/L glucose and 20 g/L galactose | (NH4)2SO4 | - | 30 | 300 rpm | - | OD = 0.05 | - | Shake-flask | [81] | |
| Limonene | 918 | 20 g/L initial glucose and 10 g/L pure ethanol | Tryptone, Yeast extract | - | 30 | 250 rpm | - | OD = 0.2 | Pure ethanol | Fed-Batch in shake flask | [72] | |
| Geraniol | 5 | 10 g/L glucose | (NH4)2SO4 | - | 28 | - | - | 2.5% | - | Shake-flask | [88] | |
| Geraniol | 293 | 20 g/L initial glucose and then fed solution (glucose and other nutrients) | (NH4)2SO4 | 6.0 | 30 | 600 rpm/1 vvm | >30% | OD = 0.15 | Fed solution (glucose and other nutrients) feeding by controlling the specific feed rate to 0.1 h−1 | Fed-Batch | [69] | |
| Geraniol | 1680 | Initial YPD medium, then glucose and ethanol | Yeast extract, Peptone | 5.7 | 30 | 300–500 rpm /2 vvm | >30% | 10% | Glucose feeding under 1 g/L and ethanol feeding under 5 g/L | Fed-Batch (Carbon restricted) | [73] | |
| Geraniol | 1690 | Initial 20 g/L glucose then pure ethanol feeding | (NH4)2SO4 | 5.0 | 30 | 600 rpm/1 vvm | >30% | OD = 0.2 | 400 g/L pure ethanol at 0.1 L/h feed rate | Fed-Batch | [69] | |
| Sabinene | 17.5 | 20 g/L glucose 20 g/L galactose and 10 g/L raffinose | (NH4)2SO4 | - | - | - | - | - | - | - | [70] | |
| (S)-linalool | 0.26 | 20 g/L glucose | Yeast extract | 5.5 | 30 | 400 rpm/2 vvm | - | OD = 0.05 | - | Batch Bioreactor | [68] | |
| Sesquiterpenes | Bisabolone | 900 | 2 g/L glucose and 18 g/L galactose | Yeast extract, peptone | - | 30 | 180 rpm | - | OD = 0.05 | - | Shake-flask | [66] |
| Bisabolone | 5200 | Initial 15 g/L glucose | (NH4)2SO4 | 5.0 | 30 | 0.7 L/min air | - | OD = 0.1 | Constant feed rate with pH rise trigger | Fed-batch | [76] | |
| Nerolidol | 336.5 | 20 g/L sucrose | (NH4)2SO4 | - | 30 | 180 rpm | - | OD = 0.2 | - | Two-phase flask cultivation | [19] | |
| Nerolidol | 5500 | Initial 20 g/L glucose, and then sucrose feeding | (NH4)2SO4 | 5.0 | - | 300–600 rpm/1.58–3.16 L/h air | 30% | OD = 0.2 | Exponential feeding (3 mM sucrose/g biomass/h) with specific increasing rate (0.05 h−1 then 20 g/L sucrose pulse feeding by DO spikes | Fed-Batch | [84] | |
| Valerenic acid | 4 | 20 g/L galactose and 2 g/L dextrose | Yeast extract, Peptone | - | 30 | 200 rpm | - | - | - | Milliliter plates | [82] | |
| Polpunonic acid | 1.4 | 20 g/L galactose and 10 g/L raffinose | - | - | 30 | 150 rpm | - | - | - | Shake-flask | [20] | |
| Amorpha-4,11-diene | >4000 | Initial 20 g/L glucose then pure ethanol pulse feeding, 0.25 g/L methionine as inducer | (NH4)2SO4 | 5.0 | 30 | 1 L/min air | 40% | - | Ethanol pulse feed (10 g/L), Off-gas CO2 evaluation rate control | Fed-Batch | [60] | |
| Artemisinic acid | 25,000 | Initial 20 g/L glucose then pure ethanol pulse feeding, 0.25 g/L methionine as inducer | (NH4)2SO4 | 5.0 | 30 | 1 L/min air | 40% | - | Etanol pulse (10 g/L) feed, stir rate control | Fed-Batch | [27] | |
| Patchoulol | 467 | Initial glucose (25 g/L) feeding, Feeds: (1) Sole glucose feeding (2) glucose/glycerol feeding, (3) Sole ethanol feeding | (NH4)2SO4, Peptone. Yeast extract | 5.5 | 30 | 200–500 rpm/1–2 vvm | - | 14% | carbon-source (glucose) controlled three-stage fermentation | Fed-Batch | [22] | |
| Zerumbone | 40 | Initial 20 g/L glucose, then feed solution (glucose, other nutrients and ingredients) | Peptone. Yeast extract | 5.5 | 30 | 300–600 rpm/2 vvm | >30% | 5% | Fed rate solution (glucose and other nutrients, ingredients) of 2 mL/min control by DO and pH rise trigger | Fed-Batch | [77] | |
| α-humulene | 1700 | Initial 20 g/L glucose, then feed solution (glucose, other nutrients and ingredients) | Peptone. Yeast extract | 5.5 | 30 | 300–600 rpm/2 vvm | >30% | 10% | Fed rate solution (glucose and other nutrients, ingredients) of 2 mL/min control by DO and pH rise trigger | Fed-Batch | [4] | |
| α-santalene | 0.036 Cmmol (g/biomass/h) | Initial 10 g/L glucose and continuous glucose and dodecane feeding | (NH4)2SO4 | 5.0 | 30 | 600 rpm/1 vvm | >30% | 2 Xi = 1 g/L | Two phase feeding (organic phase and 10 g/L glucose), dilution rates of 0.05/h and 0.1/h | Continuous | [61] | |
| Diterpenes | Geranylgeraniol | 3310 | Initial 1 g/L glucose, then sole glucose and glucose/ethanol mix. feeding | (NH4)2SO4 corn steep liquor | 5.5 | 33 | 900 rpm/1 vvm | - | - | Glucose (50% wt/v) and then glucose/ethanol (25% wt/v/50% v/v) feeding (rate of 5.8 g/h) | Fed-Batch | [89] |
| Miltiradiene | 488 | Initial 20 g/L glucose, then feed solution(glucose, other nutrients and ingredients) | Peptone. Yeast extract | 5.5 | 30 | 600 rpm/5 L/h air | - | OD = 0.05 | Feed solution (glucose and other nutrients) addition by 5 mL/h feed rate. | Fed-Batch | [78] | |
| Oxygenated taxanes | 33 | Initial 40 g/L glucose or 20 g/L xylose | (NH4)2HPO4 Yeast extract | 7.0 | 30 and 22 | 280–800 rpm/0.5 L/min | 30% | 1% for E. coli and 2% for S. cerevisiae | Pulse feeding by carbon source control (20 g/L of glucose feed when glucose below 20 g/L and 50 g/L xylose feed when xylose conc. below 10 g/L) | Fed-Batch (co-culture) | [90] | |
| Triterpenes | Protopanaxadiol and dammarenediol-II | 1189 and 1548 | Initial 25 g/L glucose and then glucose feeding | (NH4)2SO4 | 5.5 | 30 | 1000 rpm/5 L/min air | - | OD = 0.5 | Fed solution (glucose and other nutrients) addition when ethanol below 0.5 g/L | Fed-Batch | [79] |
| β-amyrin | 139 | Initial 20 g/L glucose and then pulse ethanol feeding | (NH4)2SO4 | - | 30 | 1 vvm | - | OD = 0.2 | Pulse ethanol (5 g/L) feeding at every 12 h | Fed-Batch | [71] | |
| β-amyrin | 108 | Initial 20 g/L dextrose, and then pulse glucose feeding | Yeast extract Peptone | 6.0 | 30 | - | - | - | Pulse glucose (5 mg/L) feeding at every 12 h | Fed-Batch | [85] | |
| Betulinic acid | 182 | Initial 50 g/L glucose and then pulse ethanol feeding | NH4Cl | 6.0 | 30 | 1 vvm | >30% | Xi2 = 0.08 g/L | Pulse ethanol (25 g/L) feeding control with DO spikes | Fed-Batch | [75] | |
| Ginsenoside Rh2 | 2250 | Initial 25 g/L glucose and then glucose feeding | Yeast extract Peptone | 5.0 | 30 | - | >30% | 11% | Fed solution (glucose and other nutrients) addition when ethanol below 0.5 g/L | Fed-Batch | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carsanba, E.; Pintado, M.; Oliveira, C. Fermentation Strategies for Production of Pharmaceutical Terpenoids in Engineered Yeast. Pharmaceuticals 2021, 14, 295. https://doi.org/10.3390/ph14040295
Carsanba E, Pintado M, Oliveira C. Fermentation Strategies for Production of Pharmaceutical Terpenoids in Engineered Yeast. Pharmaceuticals. 2021; 14(4):295. https://doi.org/10.3390/ph14040295
Chicago/Turabian StyleCarsanba, Erdem, Manuela Pintado, and Carla Oliveira. 2021. "Fermentation Strategies for Production of Pharmaceutical Terpenoids in Engineered Yeast" Pharmaceuticals 14, no. 4: 295. https://doi.org/10.3390/ph14040295