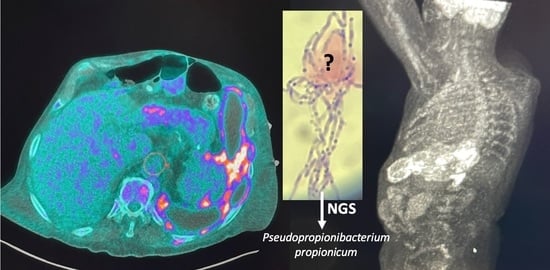
Pseudopropionibacterium propionicum as a Cause of Empyema; A Diagnosis with Next-Generation Sequencing
Abstract
:1. Introduction
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowden, G.H.W. Actinomyces, Propionibacterium propionicus, and Streptomyces. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 34. Available online: http://www.ncbi.nlm.nih.gov/books/NBK8385/ (accessed on 13 March 2023).
- Valour, F.; Sénéchal, A.; Dupieux, C.; Karsenty, J.; Lustig, S.; Breton, P.; Gleizal, A.; Boussel, L.; Laurent, F.; Braun, E.; et al. Actinomycosis: Etiology, clinical features, diagnosis, treatment, and management. Infect. Drug Resist. 2014, 7, 183. [Google Scholar] [CrossRef]
- Seal, D.V.; McGill, J.; Flanagan, D.; Purrier, B. Lacrimal canaliculitis due to Arachnia (Actinomyces) propionica. Br. J. Ophthalmol. 1981, 65, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Brazier, J.S.; Hall, V. Propionibacterium propionicum and infections of the lacrimal apparatus. Clin. Infect. Dis. 1993, 17, 892–893. [Google Scholar] [CrossRef] [PubMed]
- Novak, A.; Brütsch, P. Case report of actinomycosis caused by Arachnia propionica. Infection 1980, 8 (Suppl. 2), S209–S211. [Google Scholar] [CrossRef] [PubMed]
- Pulverer, G.; Schütt-Gerowitt, H.; Schaal, K.P. Human cervicofacial actinomycoses: Microbiological data for 1997 cases. Clin. Infect. Dis. 2003, 37, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Miglets, A.W.; Branson, D. Arachnia propionica (Actinomyces propionicus) as an unusual agent in tympanomastoiditis. Arch. Otolaryngol. 1983, 109, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Brock, D.W.; Georg, L.K.; Brown, J.M.; Hicklin, M.D. Actinomycosis caused by Arachnia propionica: Report of 11 cases. Am. J. Clin. Pathol. 1973, 59, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Karnik, A.M.; Elhag, K.M.; Fenech, F.F. Arachnia propionica pneumonia in hairy cell leukaemia. Br. J. Dis. Chest 1988, 82, 418–420. [Google Scholar] [CrossRef]
- Conrad, S.E.; Breivis, J.; Fried, M.A. Vertebral osteomyelitis, caused by Arachnia propionica and resembling actinomycosis. Report of a case. J. Bone Joint Surg. Am. 1978, 60, 549–553. [Google Scholar] [CrossRef]
- Albright, L.; Toczek, S.; Brenner, V.J.; Ommaya, A.K. Osteomyelitis and epidural abscess caused by Arachnia propionica. Case report. J. Neurosurg. 1974, 40, 115–119. [Google Scholar] [CrossRef]
- Wunderink, H.F.; Lashley, E.E.L.O.; van Poelgeest, M.I.E.; Gaarenstroom, K.N.; Claas, E.C.J.; Kuijper, E.J. Pelvic actinomycosis-like disease due to Propionibacterium propionicum after hysteroscopic removal of an intrauterine device. J. Clin. Microbiol. 2011, 49, 466–468. [Google Scholar] [CrossRef]
- Yonetani, S.; Ohnishi, H.; Araki, K.; Hiroi, M.; Takagi, Y.; Ichimura, S.; Watanabe, T. A psoas abscess caused by Propionibacterium propionicum. J. Infect. Chemother. 2014, 20, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Chau, A.M.T.; Xu, L.L.; Fairhall, J.M.; Chaganti, J.; McMullan, B.J. Brain abscess due to Propionibacterium propionicum in Eisenmenger syndrome. Med. J. Aust. 2012, 196, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Riley, T.V.; Ott, A.K. Brain abscess due to Arachnia propionica. Br. Med. J. Clin. Res. Ed. 1981, 282, 1035. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Arshava, E.V.; Ford, B.; Nauseef, W.M. Don’t let its name fool you: Relapsing thoracic actinomycosis caused by Pseudopropionibacterium propionicum (formerly Propionibacterium propionicum). Am. J. Case Rep. 2019, 20, 1961–1965. [Google Scholar] [CrossRef] [PubMed]
- Charfreitag, O.; Collins, M.D.; Stackebrandt, E. Reclassification of Arachnia propionica as Propionibacterium propionicus comb. nov. Int. J. Syst. Evol. Microbiol. 1988, 38, 354–357. [Google Scholar] [CrossRef]
- Pine, L.; Georg, L.K. Reclassification of Actinomyces propionicus. Int. J. Syst. Evol. Microbiol. 1969, 19, 267–272. [Google Scholar] [CrossRef]
- Breed, R.S.; Conn, H.J. The nomenclature of the Actinomycetaeae. J. Bacteriol. 1919, 4, 585–602. [Google Scholar] [CrossRef]
- de la Maza, L.; Pezzlo, M.T.; Baron, E.J. Color Atlas of Diagnostic Microbiology; Mosby: St. Louis, MO, USA, 1997. [Google Scholar]
- Chandler, F.W. Approaches to the pathologic diagnosis of infectious diseases. In Pathology of Infectious Diseases; Connor, D.H., Chandler, F.W., Manz, H.J., Schwartz, D.A., Lack, E.E., Eds.; Appleton and Lange: Stamford, CT, USA, 1997; pp. 3–7. [Google Scholar]
- Kumar, A.; Gupta, K. Challenge of a false-positive acid-fast bacilli: A diagnostic conundrum. J. R. Coll. Physicians Edinb. 2021, 51, 369–372. [Google Scholar] [CrossRef]
- Lowe, R.N.; Azimi, P.H.; McQuitty, J. Acid-fast actinomyces in a child with pulmonary actinomycosis. J. Clin. Microbiol. 1980, 12, 124–126. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, E.C.; Joo, S.I.; Lee, S.M.; Yoo, C.G.; Kim, Y.W.; Han, S.K.; Shim, Y.S.; Yim, J.J. The incidence and clinical implication of sputum with positive acid-fast bacilli smear but negative in mycobacterial culture in a tertiary referral hospital in South Korea. J. Korean Med. Sci. 2008, 23, 767–771. [Google Scholar] [CrossRef]
- Bao, J.R.; Master, R.N.; Schwab, D.A.; Clark, R.B. Identification of acid-fast bacilli using pyrosequencing analysis. Diagn. Microbiol. Infect. Dis. 2010, 67, 234–238. [Google Scholar] [CrossRef]
- Quest Diagnostics. Acid Fast Bacillus AFB Identification Sequencing and Stain Paraffin Block. Available online: https://www.questdiagnostics.com/healthcare-professionals/clinical-education-center/faq/faq64 (accessed on 18 November 2023).
- Ben-Ezra, J.; Johnson, D.A.; Rossi, J.; Cook, N.; Wu, A. Effect of fixation on the amplification of nucleic acids from paraffin-embedded material by the polymerase chain reaction. J. Histochem. Cytochem. 1991, 39, 351–354. [Google Scholar] [CrossRef]
- Rasool, G.; Khan, A.M.; Mohy-Ud-Din, R.; Riaz, M. Detection of Mycobacterium tuberculosis in AFB smear-negative sputum specimens through MTB culture and GeneXpert® MTB/RIF assay. Int. J. Immunopathol. Pharmacol. 2019, 33, 1–6. [Google Scholar] [CrossRef]
- Bertrán-López, J.; Abbott, A.; Archibald, L.K.; Benninger, L.; Lascano, J.; Kalyatanda, G. Disseminated Nocardia beijingensis masquerading as pulmonary tuberculosis in a patient with Human Immunodeficiency Virus/Acquired Immune Deficiency Syndrome. Open Forum Infect. Dis. 2020, 7, ofaa186. [Google Scholar] [CrossRef] [PubMed]
- Behera, H.S.; Chayani, N.; Bal, M.; Kuntia, K.H.; Pati, S.; Das, S.; Ranjit, M. Identification of population of bacteria from culture negative surgical site infection patients using molecular tool. BMC Surg. 2021, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Miller, S.; Chiu, C.Y. Clinical metagenomic next-generation sequencing for pathogen detection. Annu. Rev. Pathol. 2019, 14, 319–338. [Google Scholar] [CrossRef]
- MicroGenDX. Available online: https://microgendx.com/ (accessed on 2 December 2023).
- Forbes, J.D.; Knox, N.C.; Ronholm, J.; Pagotto, F.; Reimer, A. Metagenomics: The next culture-independent game changer. Front. Microbiol. 2017, 8, 1069. [Google Scholar] [CrossRef]
- Tanaka-Bandoh, K.; Watanabe, K.; Kato, N.; Ueno, K. Susceptibilities of Actinomyces species and Propionibacterium propionicus to antimicrobial agents. Clin. Infect. Dis. 1997, 25 (Suppl. 2), S262–S263. [Google Scholar] [CrossRef]
- Mabeza, G.F.; Macfarlane, J. Pulmonary actinomycosis. Eur. Respir. J. 2003, 21, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Peiffer-Smadja, N.; Harent, S.; Messeca, C.; Lechapt-Zalcman, E.; Yazdanpanah, Y.; Joly, V. A case of thoracic actinomycosis presenting as sudden paraplegia. Rev. Neurol. 2019, 175, 89–92. [Google Scholar] [CrossRef] [PubMed]






| Day | Clinical Events |
|---|---|
| −59 |
|
| −39 |
|
| 1 |
|
| 2 |
|
| 4 |
|
| 8 |
|
| 11 |
|
| 12 |
|
| 19 |
|
| 24 |
|
| 36 |
|
| 38 |
|
| 46 |
|
| 47 |
|
| 279 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babar, S.; Liu, E.; Kaur, S.; Hussain, J.; Danaher, P.J.; Anstead, G.M. Pseudopropionibacterium propionicum as a Cause of Empyema; A Diagnosis with Next-Generation Sequencing. Pathogens 2024, 13, 165. https://doi.org/10.3390/pathogens13020165
Babar S, Liu E, Kaur S, Hussain J, Danaher PJ, Anstead GM. Pseudopropionibacterium propionicum as a Cause of Empyema; A Diagnosis with Next-Generation Sequencing. Pathogens. 2024; 13(2):165. https://doi.org/10.3390/pathogens13020165
Chicago/Turabian StyleBabar, Sumbal, Emily Liu, Savreet Kaur, Juzar Hussain, Patrick J. Danaher, and Gregory M. Anstead. 2024. "Pseudopropionibacterium propionicum as a Cause of Empyema; A Diagnosis with Next-Generation Sequencing" Pathogens 13, no. 2: 165. https://doi.org/10.3390/pathogens13020165