Inferring the Potential Distribution of an Emerging Rickettsiosis in America: The Case of Rickettsia parkeri
Abstract
:1. Introduction
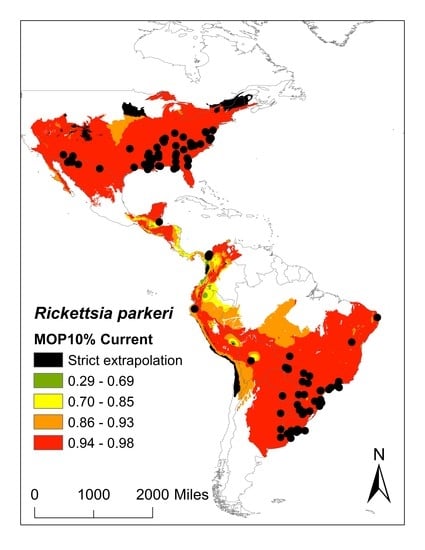
2. Results
3. Discussion
4. Materials and Methods
4.1. Database and Accessible Area M
4.2. Bioclim Variables
4.3. ENM
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sánchez-Montes, S.; Colunga-Salas, P.; Lozano-Sardaneta, Y.N.; Zazueta-Islas, H.M.; Ballados-González, G.G.; Salceda-Sánchez, B.; Huerta-Jiménez, H.; Torres-Castro, M.; Panti-May, J.A.; Peniche-Lara, G.; et al. The genus Rickettsia in Mexico: Current knowledge and perspectives. Ticks Tick-Borne Dis. 2021, 12, 101633. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the World: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labruna, M.B.; Salim-Mattar, V.; Nava, S.; Bermúdez, S.; Venzal, J.; Dolz, G.; Abarca, K.; Romero, L.; de Souza, R.; Oteo, J.; et al. Rickettsiosis in Latin America, Caribbean, Spain and Portugal. Rev. MVZ Cordoba 2011, 16, 2435–2457. [Google Scholar] [CrossRef] [Green Version]
- Parker, R.R.; Kohls, G.M.; Cox, G.W.; Davis, G.E. Observations on an infectious agent from Amblyomma maculatum. Public Health Rep. 1939, 54, 1482. [Google Scholar] [CrossRef]
- Paddock, C.D.; Sumner, J.W.; Comer, J.A.; Zaki, S.R.; Goldsmith, C.S.; Goddard, J.; McLellan, S.L.F.; Tamminga, C.L.; Ohl, C.A. Rickettsia parkeri: A newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 2004, 38, 805–811. [Google Scholar] [CrossRef] [Green Version]
- Conti-Díaz, I.A.; Moraes-Filho, J.; Pacheco, R.C.; Labruna, M.B. Serological evidence of Rickettsia parkeri as the etiological agent of rickettsiosis in Uruguay. Revista do Instituto de Medicina Tropical de São Paulo 2009, 51, 337–339. [Google Scholar] [CrossRef] [Green Version]
- Spolidorio, M.G.; Labruna, M.B.; Mantovani, E.; Brandão, P.E.; Richtzenhain, L.J.; Yoshinari, N.H. Novel spotted fever group rickettsiosis, Brazil. Emerg. Infect. Dis. 2010, 16, 521–523. [Google Scholar] [CrossRef]
- Romer, Y. Rickettsia parkeri Rickettsiosis, Argentina. Emerg. Infect. Dis. 2011, 17, 1169–1173. [Google Scholar] [CrossRef]
- Eisen, R.J.; Kugeler, K.J.; Eisen, L.; Beard, C.B.; Paddock, C.D. Tick-borne zoonoses in the United States: Persistent and emerging threats to human health. ILAR J. 2017, 58, 319–335. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, A.R.; Filho, J.M.; Nieri-Bastos, F.A.; Souza, J.C.; Szabó, M.P.; Labruna, M.B. Epidemiology of Rickettsia sp. strain Atlantic rainforest in a spotted fever-endemic area of southern Brazil. Ticks Tick-Borne Dis. 2014, 5, 848–853. [Google Scholar] [CrossRef]
- Nieri-Bastos, F.A.; Horta, M.C.; Barros-Battesti, D.M.; Moraes-Filho, J.; Ramirez, D.G.; Martins, T.F.; Labruna, M.B. Isolation of the pathogen Rickettsia sp. strain Atlantic Rainforest from its presumed tick vector, Amblyomma ovale (Acari: Ixodidae), from two areas of Brazil. J. Med. Éntomol. 2016, 53, 977–981. [Google Scholar] [CrossRef]
- Paddock, C.D.; Allerdice, M.E.J.; Karpathy, S.E.; Nicholson, W.L.; Levin, M.L.; Smith, T.C.; Becker, T.; Delph, R.J.; Knight, R.; Ritter, J.M.; et al. Unique strain of Rickettsia parkeri associated with the hard tick Dermacentor parumapertus Neumann in the Western United States. Appl. Environ. Microbiol. 2017, 83, e03463-16. [Google Scholar] [CrossRef] [Green Version]
- Nieri-Bastos, F.A.; Marcili, A.; De Sousa, R.; Paddock, C.D.; Labruna, M.B. Phylogenetic evidence for the existence of multiple strains of Rickettsia parkeri in the New World. Appl. Environ. Microbiol. 2018, 84, e02872-17. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Montes, S.; López-Pérez, A.M.; Guzmán-Cornejo, C.; Colunga-Salas, P.; Becker, I.; Delgado-de la Mora, J.; Licona-Enríquez, J.D.; Delgado-de la Mora, D.; Karpathy, S.E.; Paddock, C.D.; et al. Rickettsia parkeri in Dermacentor parumapertus ticks. Mexico. Emerg. Infect. Dis. 2018, 24, 1108–1111. [Google Scholar] [CrossRef] [Green Version]
- Tomassone, L.; Conte, V.; Parrilla, G.; De Meneghi, D. Rickettsia infection in dogs and Rickettsia parkeri in Amblyomma tigrinum ticks, Cochabamba Department, Bolivia. Vector-Borne Zoonotic Dis. 2010, 10, 953–958. [Google Scholar] [CrossRef] [Green Version]
- Lopes, M.G.; Junior, J.M.; Foster, R.J.; Harmsen, B.J.; Sanchez, E.; Martins, T.F.; Quigley, H.; Marcili, A.; Labruna, M.B. Ticks and rickettsiae from wildlife in Belize, Central America. Parasites Vectors 2016, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- La Mora, J.D.-D.; Sánchez-Montes, S.; Licona-Enríquez, J.D.; La Mora, D.D.-D.; Paddock, C.D.; Beati, L.; Colunga-Salas, P.; Guzmán-Cornejo, C.; Zambrano, M.L.; Karpathy, S.E.; et al. Rickettsia parkeri and Candidatus Rickettsia andeanae in tick of the Amblyomma maculatum group, Mexico. Emerg. Infect. Dis. 2019, 25, 836–838. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2012; ISBN 978-0-691-13686-8. [Google Scholar]
- Moo-Llanes, D.; Ibarra-Cerdeña, C.N.; Rebollar-Tellez, E.A.; Ibáñez-Bernal, S.; Gonzalez, C.; Ramsey, J.M. Current and future niche of North and Central American sand flies (Diptera: Psychodidae) in climate change scenarios. PLoS Negl. Trop. Dis. 2013, 7, e2421. [Google Scholar] [CrossRef] [Green Version]
- Moo-Llanes, D.A.; Pech-May, A.; Ibarra-Cerdeña, C.N.; Rebollar-Téllez, E.A.; Ramsey, J.M. Inferring distributional shifts of epidemiologically important North and Central American sandflies from Pleistocene to future scenarios. Med. Vet. Éntomol. 2019, 33, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Pech-May, A.; Moo-Llanes, D.A.; Puerto-Avila, M.B.; Casas, M.; Danis-Lozano, R.; Ponce, G.; Tun-Ku, E.; Pinto-Castillo, J.F.; Villegas, A.; Ibáñez-Piñon, C.R.; et al. Population genetics and ecological niche of invasive Aedes albopictus in Mexico. Acta Trop. 2016, 157, 30–41. [Google Scholar] [CrossRef]
- Moo-Llanes, D.; López-Ordóñez, T.; Torres-Monzón, J.; Mosso-González, C.; Casas-Martínez, M.; Samy, A. Assessing the potential distributions of the invasive mosquito vector Aedes albopictus and its natural Wolbachia infections in Mexico. Insects 2021, 12, 143. [Google Scholar] [CrossRef]
- Ogrzewalska, M.; Nieri-Bastos, F.A.; Marcili, A.; Nava, S.; González-Acuña, D.; Muñoz-Leal, S.; Ruiz-Arrondo, I.; Venzal, J.M.; Mangold, A.; Labruna, M.B. A novel spotted fever group Rickettsia infecting Amblyomma parvitarsum (Acari: Ixodidae) in highlands of Argentina and Chile. Ticks Tick-Borne Dis. 2016, 7, 439–442. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef] [Green Version]
- John, H.K.S.; Adams, M.L.; Masuoka, P.M.; Flyer-Adams, J.G.; Jiang, J.; Rozmajzl, P.J.; Stromdahl, E.Y.; Richards, A.L. Prevalence, Distribution, and development of an ecological niche model of Dermacentor variabilis ticks positive for Rickettsia montanensis. Vector-Borne Zoonotic Dis. 2016, 16, 253–263. [Google Scholar] [CrossRef]
- Raghavan, R.K.; Peterson, A.T.; Cobos, M.E.; Ganta, R.; Foley, D. Current and future distribution of the lone star tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS ONE 2019, 14, e0209082. [Google Scholar] [CrossRef]
- Pascoe, E.L.; Marcantonio, M.; Caminade, C.; Foley, J.E. Modeling potential habitat for Amblyomma tick species in California. Insects 2019, 10, 201. [Google Scholar] [CrossRef] [Green Version]
- Lippi, C.A.; Gaff, H.D.; White, A.L.; Ryan, S.J. Scoping review of distribution models for selected Amblyomma ticks and rickettsial group pathogens. PeerJ 2021, 9, e10596. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jiménez-Valverde, A.; Lira-Noriega, A.; Maher, S.P.; Peterson, A.T.; Soberón, J.; Villalobos, F. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. Model. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on earth. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Escobar, L.E.; Lira-Noriega, A.; Medina-Vogel, G.; Peterson, A.T. Potential for spread of the white-nose fungus (Pseudogymnoascus destructans) in the Americas: Use of Maxent and NicheA to assure strict model transference. Geospat. Health 2014, 9, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Rödder, D.; Schmidtlein, S.; Veith, M.; Lötters, S. Alien invasive slider turtle in unpredicted habitat: A matter of niche shift or of predictors studied? PLoS ONE 2009, 4, e7843. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Aguilar-Domínguez, A.; Moo-Llanes, D.A.; Sánchez-Montes, S.; Becker, I.; Feria-Arroyo, T.; de León, A.P.; Romero-Salas, D. Potential distribution of Amblyomma mixtum (Koch 1844) in climate change scenarios in America. Tick Tick-Borne Dis. 2021, in press. [Google Scholar]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef]



| Best Models | RM | FC * | p.ROC | O.rate 5% | AICc | ∆AICc | AICc.W | # |
|---|---|---|---|---|---|---|---|---|
| 1th | 4 | p | 1.06 | 0.03 | 2623.39 | 0.00 | 0.81 | 5 |
| 2th | 4 | qp | 1.06 | 0.03 | 2623.42 | 0.03 | 0.43 | 5 |
| 3th | 5 | lqpt | 1.06 | 0.00 | 2624.43 | 1.03 | 0.14 | 7 |
| 4th | 5 | lqpth | 1.06 | 0.00 | 2624.43 | 1.03 | 0.10 | 7 |
| 5th | 5 | lpt | 1.05 | 0.00 | 2624.51 | 1.12 | 0.16 | 7 |
| 6th | 5 | lpth | 1.05 | 0.00 | 2624.51 | 1.12 | 0.11 | 7 |
| 7th | 6 | lqpt | 1.05 | 0.00 | 2625.01 | 1.62 | 0.06 | 5 |
| 8th | 6 | lqpth | 1.05 | 0.00 | 2625.01 | 1.62 | 0.05 | 5 |
| 9th | 6 | lpt | 1.05 | 0.00 | 2625.02 | 1.63 | 0.06 | 5 |
| 10th | 6 | lpth | 1.05 | 0.00 | 2625.02 | 1.63 | 0.05 | 5 |
| Times | RCP2.6 | RCP4.5 | RCP6.0 | RCP8.5 | SSP126 | SSP245 | SSP370 | SSP585 |
|---|---|---|---|---|---|---|---|---|
| Current | 0.836 | 0.841 | 0.839 | 0.836 | 0.964 | 0.964 | 0.964 | 0.964 |
| RCP2.6 | 0.956 | 0.954 | 0.955 | 0.834 | 0.834 | 0.834 | 0.834 | |
| RCP4.5 | 0.95 | 0.96 | 0.842 | 0.842 | 0.842 | 0.842 | ||
| RCP6.0 | 0.954 | 0.839 | 0.839 | 0.839 | 0.839 | |||
| RCP8.5 | 0.837 | 0.837 | 0.837 | 0.837 | ||||
| SSPS126 | 1.000 | 1.000 | 1.000 | |||||
| SPSS245 | 1.000 | 1.000 | ||||||
| SPSS370 | 1.000 |
| Bioclim Variables | Code | set1 | set2 | set3 |
|---|---|---|---|---|
| Annual Mean Temperature | Bio01 | X | X | X |
| Mean Diurnal Range | Bio02 | X | ||
| Isothermality (BIO2/BIO7) (×100) | Bio03 | X | ||
| Temperature Seasonality (standard deviation × 100) | Bio04 | X | X | X |
| Max Temperature of Warmest Month | Bio05 | X | X | |
| Min Temperature of Coldest Month | Bio06 | X | X | X |
| Temperature Annual Range (BIO5-BIO6) | Bio07 | X | X | X |
| Mean Temperature of Warmest Quarter | Bio10 | X | ||
| Mean Temperature of Coldest Quarter | Bio11 | X | ||
| Annual Precipitation | Bio12 | X | X | |
| Precipitation of Wettest Month | Bio13 | X | X | |
| Precipitation of Driest Month | Bio14 | X | X | X |
| Precipitation Seasonality (Coefficient of Variation) | Bio15 | X | X | X |
| Precipitation of Wettest Quarter | Bio16 | X | ||
| Precipitation of Driest Quarter | Bio17 | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moo-Llanes, D.A.; Oca-Aguilar, A.C.M.d.; Romero-Salas, D.; Sánchez-Montes, S. Inferring the Potential Distribution of an Emerging Rickettsiosis in America: The Case of Rickettsia parkeri. Pathogens 2021, 10, 592. https://doi.org/10.3390/pathogens10050592
Moo-Llanes DA, Oca-Aguilar ACMd, Romero-Salas D, Sánchez-Montes S. Inferring the Potential Distribution of an Emerging Rickettsiosis in America: The Case of Rickettsia parkeri. Pathogens. 2021; 10(5):592. https://doi.org/10.3390/pathogens10050592
Chicago/Turabian StyleMoo-Llanes, David A., Ana C. Montes de Oca-Aguilar, Dora Romero-Salas, and Sokani Sánchez-Montes. 2021. "Inferring the Potential Distribution of an Emerging Rickettsiosis in America: The Case of Rickettsia parkeri" Pathogens 10, no. 5: 592. https://doi.org/10.3390/pathogens10050592