Ethanol Extract of Licorice Alleviates HFD-Induced Liver Fat Accumulation in Association with Modulation of Gut Microbiota and Intestinal Metabolites in Obesity Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Licorice Ethanol Extract (LEE) Preparation
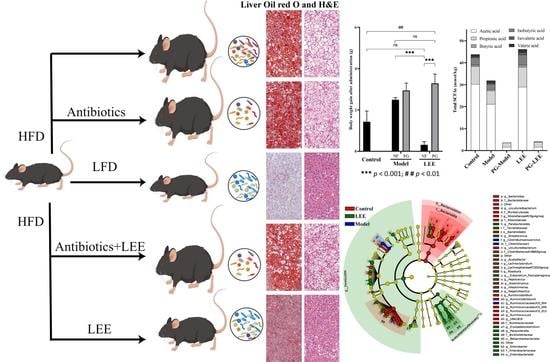
2.2. Animal, Diets, and Experimental Design
2.3. Oral Glucose Tolerance Test (OGTT)
2.4. Histological Analysis
2.5. Biochemical Analysis, Endotoxemia and Cytokine Measurement
2.6. Real-Time PCR Analysis
2.7. Measurement of Short-Chain Fatty Acids (SCFAs) Concentration in the Colon Digested
2.8. Gut Microbiota Analysis
2.9. Cell Experiment
2.10. Statistical Analysis
3. Results
3.1. LEE Administration Attenuated the Obesity Characteristics of HFD Mice
3.2. Effects of LEE on Serum Lipid Profile and Glucose Tolerance
3.3. LEE Treatment Reduces HFD-Induced Liver Fat Accumulation and Damage
3.4. LEE Treatment Changes the Composition of Gut Microbiota in HFD Mice
3.5. Effect of LEE on Pseudo-Germ-Free Mice with HFD
3.6. LEE Modulates HFD-Induced Abnormal Hepatic Lipid Synthesis and Inflammatory Factors
3.7. Impact of LEE Administration on Colonic SCFA Metabolism
3.8. Correlation Analysis of Feature Genera and Metabolic Parameters
3.9. Effects of GL, GA and LEE on FFA-Induced HepG2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Burhans, M.S.; Flowers, M.T.; Ntambi, J.M. Hepatic oleate regulates liver stress response partially through PGC-1alpha during high-carbohydrate feeding. J. Hepatol. 2016, 65, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Watt, K.I.; Henstridge, D.C.; Ziemann, M.; Sim, C.B.; Montgomery, M.K.; Samocha-Bonet, D.; Parker, B.L.; Dodd, G.T.; Bond, S.T.; Salmi, T.M.; et al. Yap regulates skeletal muscle fatty acid oxidation and adiposity in metabolic disease. Nat. Commun. 2021, 12, 2887. [Google Scholar] [CrossRef]
- Item, F.; Wueest, S.; Lemos, V.; Stein, S.; Lucchini, F.C.; Denzler, R.; Fisser, M.C.; Challa, T.D.; Pirinen, E.; Kim, Y.; et al. Fas cell surface death receptor controls hepatic lipid metabolism by regulating mitochondrial function. Nat. Commun. 2017, 8, 480. [Google Scholar] [CrossRef] [Green Version]
- Atabaki-Pasdar, N.; Ohlsson, M.; Vinuela, A.; Frau, F.; Pomares-Millan, H.; Haid, M.; Jones, A.G.; Thomas, E.L.; Koivula, R.W.; Kurbasic, A.; et al. Predicting and elucidating the etiology of fatty liver disease: A machine learning modeling and validation study in the IMI DIRECT cohorts. PLoS Med. 2020, 17, e1003149. [Google Scholar] [CrossRef]
- Hasin-Brumshtein, Y.; Sakaram, S.; Khatri, P.; He, Y.D.; Sweeney, T.E. A robust gene expression signature for NASH in liver expression data. Sci. Rep. 2022, 12, 2571. [Google Scholar] [CrossRef]
- Carpene, C.; Pejenaute, H.; Del Moral, R.; Boulet, N.; Hijona, E.; Andrade, F.; Villanueva-Millan, M.J.; Aguirre, L.; Arbones-Mainar, J.M. The Dietary Antioxidant Piceatannol Inhibits Adipogenesis of Human Adipose Mesenchymal Stem Cells and Limits Glucose Transport and Lipogenic Activities in Adipocytes. Int. J. Mol. Sci. 2018, 19, 2081. [Google Scholar] [CrossRef] [Green Version]
- Heendeniya, S.N.; Keerthirathna, L.R.; Manawadu, C.K.; Dissanayake, I.H.; Ali, R.; Mashhour, A.; Alzahrani, H.; Godakumbura, P.; Boudjelal, M.; Peiris, D.C. Therapeutic Efficacy of Nyctanthes arbor-tristis Flowers to Inhibit Proliferation of Acute and Chronic Primary Human Leukemia Cells, with Adipocyte Differentiation and in Silico Analysis of Interactions between Survivin Protein and Selected Secondary Metabolites. Biomolecules 2020, 10, 165. [Google Scholar] [CrossRef] [Green Version]
- Gou, S.H.; Liu, J.; He, M.; Qiang, Y.; Ni, J.M. Quantification and bio-assay of alpha-glucosidase inhibitors from the roots of Glycyrrhiza uralensis Fisch. Nat. Prod. Res. 2016, 30, 2130–2134. [Google Scholar] [CrossRef]
- Li, X.; Sun, R.; Liu, R. Natural products in licorice for the therapy of liver diseases: Progress and future opportunities. Pharmacol. Res. 2019, 144, 210–226. [Google Scholar] [CrossRef]
- Fan, Y.H.; Ye, R.; Xu, H.Y.; Feng, X.H.; Ma, C.M. Structures and In Vitro Antihepatic Fibrosis Activities of Prenylated Dihydrostilbenes and Flavonoids from Glycyrrhiza uralensis Leaves. J. Food Sci. 2019, 84, 1224–1230. [Google Scholar] [CrossRef]
- Kuang, Y.; Lin, Y.; Li, K.; Song, W.; Ji, S.; Qiao, X.; Zhang, Q.; Ye, M. Screening of hepatoprotective compounds from licorice against carbon tetrachloride and acetaminophen induced HepG2 cells injury. Phytomedicine 2017, 34, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.Y.; Hu, Q.; Zhu, S.N.; Jia, L.L.; Xiao, J.; Su, H.Z.; Huang, S.Y.; Zhang, J.; Jin, J. Licorice root extract and magnesium isoglycyrrhizinate protect against triptolide-induced hepatotoxicity via up-regulation of the Nrf2 pathway. Drug Deliv. 2018, 25, 1213–1223. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.; Yin, S.; Zhao, S.; Zhao, C.; Yan, M.; Fan, L.; Hu, H. Protective effects of glycycoumarin on liver diseases. Phytother. Res. 2020, 34, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Koyama, M.; Shirahata, T.; Hirashima, R.; Kobayashi, Y.; Itoh, T.; Fujiwara, R. Inhibition of UDP-glucuronosyltransferase (UGT)-mediated glycyrrhetinic acid 3-O-glucuronidation by polyphenols and triterpenoids. Drug Metab. Pharmacokinet. 2017, 32, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.; Domingues, F.; Pereira, L. Metabolic changes after licorice consumption: A systematic review with meta-analysis and trial sequential analysis of clinical trials. Phytomedicine 2018, 39, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hou, H.; Wang, X.; Dai, X.; Zhang, W.; Tang, Q.; Dong, Y.; Yan, C.; Wang, B.; Li, Z.; et al. Diammonium Glycyrrhizinate Ameliorates Obesity Through Modulation of Gut Microbiota-Conjugated BAs-FXR Signaling. Front. Pharmacol. 2021, 12, 796590. [Google Scholar] [CrossRef]
- Guo, L.; Katiyo, W.; Lu, L.; Zhang, X.; Wang, M.; Yan, J.; Ma, X.; Yang, R.; Zou, L.; Zhao, W. Glycyrrhetic Acid 3-O-Mono-beta-d-glucuronide (GAMG): An Innovative High-Potency Sweetener with Improved Biological Activities. Compr. Rev. Food Sci. Food Saf. 2018, 17, 905–919. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Ji, H.-F. Reciprocal interactions between resveratrol and gut microbiota deepen our understanding of molecular mechanisms underlying its health benefits. Trends Food Sci. Tech. 2018, 81, 232–236. [Google Scholar] [CrossRef]
- Bess, E.N.; Bisanz, J.E.; Yarza, F.; Bustion, A.; Rich, B.E.; Li, X.; Kitamura, S.; Waligurski, E.; Ang, Q.Y.; Alba, D.L.; et al. Genetic basis for the cooperative bioactivation of plant lignans by Eggerthella lenta and other human gut bacteria. Nat. Microbiol. 2020, 5, 56–66. [Google Scholar] [CrossRef]
- Wang, G.; Yu, Y.; Garcia-Gutierrez, E.; Jin, X.; He, Y.; Wang, L.; Tian, P.; Liu, Z.; Zhao, J.; Zhang, H.; et al. Lactobacillus acidophilus JCM 1132 Strain and Its Mutant with Different Bacteriocin-Producing Behaviour Have Various in Situ Effects on the Gut Microbiota of Healthy Mice. Microorganisms 2019, 8, 49. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilan, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Yang, H.J.; Park, K.I.; Ma, J.Y. Lycopus lucidus Turcz. ex Benth. Attenuates free fatty acid-induced steatosis in HepG2 cells and non-alcoholic fatty liver disease in high-fat diet-induced obese mice. Phytomedicine 2019, 55, 14–22. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Hu, W.; Liang, Y.; Zheng, L.; Zheng, J.; Wang, B.; Guo, X. Different Effects of Leucine Supplementation and/or Exercise on Systemic Insulin Sensitivity in Mice. Front. Endocrinol. 2021, 12, 651303. [Google Scholar] [CrossRef] [PubMed]
- Singer-Englar, T.; Barlow, G.; Mathur, R. Obesity, diabetes, and the gut microbiome: An updated review. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 3–15. [Google Scholar] [CrossRef]
- Thomas, K.S.; Owen, K.A.; Conger, K.; Llewellyn, R.A.; Bouton, A.H.; Casanova, J.E. Non-redundant functions of FAK and Pyk2 in intestinal epithelial repair. Sci. Rep. 2019, 9, 4497. [Google Scholar] [CrossRef] [Green Version]
- Si, H.; Yang, Q.; Hu, H.; Ding, C.; Wang, H.; Lin, X. Colorectal cancer occurrence and treatment based on changes in intestinal flora. Semin. Cancer. Biol. 2021, 70, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Xu, J.; Li, G.; Liu, T.; Guo, X.; Wang, H.; Luo, L. Ethanol extract of propolis prevents high-fat diet-induced insulin resistance and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2020, 130, 108939. [Google Scholar] [CrossRef]
- Zhao, W.; Song, F.; Hu, D.; Chen, H.; Zhai, Q.; Lu, W.; Zhao, J.; Zhang, H.; Chen, W.; Gu, Z.; et al. The Protective Effect of Myristica fragrans Houtt. Extracts Against Obesity and Inflammation by Regulating Free Fatty Acids Metabolism in Nonalcoholic Fatty Liver Disease. Nutrients 2020, 12, 2507. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhao, Y.; Chen, X.; Zhou, H.; Yang, Y.; Zhang, X.; Huang, Y.; Zhang, N.; Lui, E.M.K.; Xiao, M. Pu-erh tea ameliorates obesity and modulates gut microbiota in high fat diet fed mice. Food Res. Int. 2021, 144, 110360. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.J.; Lee, Y.K.; Ting, N.C.; Chen, Y.L.; Shen, S.C.; Wu, S.J.; Huang, W.C. Protective Effects of Licochalcone A Ameliorates Obesity and Non-Alcoholic Fatty Liver Disease Via Promotion of the Sirt-1/AMPK Pathway in Mice Fed a High-Fat Diet. Cells 2019, 8, 447. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.; Hwang, J.M.; Kang, E.J.; Kim, H.M.; Yoon, S.W.; Chung, H.C.; Lee, J.H. Anti-Obesity Effect of Licorice Acetone Extract in a Mouse Model of Obesity Induced by a High-Fat Diet. Food Suppl. Biomater. Health 2021, 1, e8. [Google Scholar] [CrossRef]
- Wang, H.F.; Li, K.; Zhang, H.; Zhang, R.X.; Zhang, Y.Q.; Wang, D.; Cheng, X.X.; Yang, F.R.; Zhang, Q.; Xue, Z.Y.; et al. Plumbagin protects liver against fulminant hepatic failure and chronic liver fibrosis via inhibiting inflammation and collagen production. Oncotarget 2016, 7, 82864–82875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.; Hydes, T.; Hamid, A.; Cuthbertson, D.J. Emerging and Established Therapeutic Approaches for Nonalcoholic Fatty Liver Disease. Clin. Ther. 2021, 43, 1476–1504. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xu, S.; Guo, H.; Lu, L.; Liu, J.; Wang, G.; Hao, K. Magnesium isoglycyrrhizinate prevents the nonalcoholic hepatic steatosis via regulating energy homeostasis. J. Cell. Mol. Med. 2020, 24, 7201–7213. [Google Scholar] [CrossRef] [PubMed]
- Madak-Erdogan, Z.; Gong, P.; Zhao, Y.C.; Xu, L.; Wrobel, K.U.; Hartman, J.A.; Wang, M.; Cam, A.; Iwaniec, U.T.; Turner, R.T.; et al. Dietary licorice root supplementation reduces diet-induced weight gain, lipid deposition, and hepatic steatosis in ovariectomized mice without stimulating reproductive tissues and mammary gland. Mol. Nutr. Food Res. 2016, 60, 369–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Lu, W.; Jin, Z.; Yu, J.; Shi, B. Carbenoxolone ameliorates hepatic lipid metabolism and inflammation in obese mice induced by high fat diet via regulating the JAK2/STAT3 signaling pathway. Int. Immunopharmacol. 2019, 74, 105498. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Peng, H.C.; Chien, Y.W.; Chen, Y.L.; Lu, N.S.; Yang, S.C. Effects of Fish Oil on Lipid Metabolism and Its Molecular Biological Regulators in Chronic Ethanol-Fed Rats. Nutrients 2018, 10, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khateeb, S.; Albalawi, A.; Alkhedaide, A. Regulatory effect of diosgenin on lipogenic genes expression in high-fat diet-induced obesity in mice. Saudi J. Biol. Sci. 2021, 28, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gu, X.; Zhang, N.; Kolonin, M.G.; An, Z.; Sun, K. Divergent functions of endotrophin on different cell populations in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E952–E963. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Li, S.; You, J.; Wang, Z.; Liu, Y.; Wang, B.; Du, M.; Zou, T. Curcumin alleviates high-fat diet-induced hepatic steatosis and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2021, 143, 110270. [Google Scholar] [CrossRef]
- Kauna, C.J.; Gątarek, P.; Chartrand, M.S.; Dadar, M.; Bjørklund, G. Is there a relationship between intestinal microbiota, dietary compounds, and obesity? Trends. Food Sci. Tech. 2017, 70, 105–113. [Google Scholar] [CrossRef]
- Wu, W.K.; Ivanova, E.A.; Orekhov, A.N. Gut microbiome: A possible common therapeutic target for treatment of atherosclerosis and cancer. Semin. Cancer Biol. 2021, 70, 85–97. [Google Scholar] [CrossRef]
- Xu, J.; Liu, T.; Li, Y.; Liu, W.; Ding, Z.; Ma, H.; Seeram, N.P.; Mu, Y.; Huang, X.; Li, L. Jamun (Eugenia jambolana Lam.) Fruit Extract Prevents Obesity by Modulating the Gut Microbiome in High-Fat-Diet-Fed Mice. Mol. Nutr. Food Res. 2019, 63, e1801307. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Dai, Z.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Fuzhuan Brick Tea Polysaccharides Attenuate Metabolic Syndrome in High-Fat Diet Induced Mice in Association with Modulation in the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 2783–2795. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Dai, Z.; Wan, P.; Ye, H.; Zeng, X.; Sun, Y. Kudingcha and Fuzhuan Brick Tea Prevent Obesity and Modulate Gut Microbiota in High-Fat Diet Fed Mice. Mol. Nutr. Food Res. 2018, 62, e1700485. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, L.; Li, Y.; Xia, G.; Chen, C.; Zhang, Y. Bamboo-shaving polysaccharide protects against high-diet induced obesity and modulates the gut microbiota of mice. J. Funct. Foods 2018, 49, 20–31. [Google Scholar] [CrossRef]
- Tremaroli, V.; Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Zhao, R.; Hu, Q.; Ma, G.; Su, A.; Xie, M.; Li, X.; Chen, G.; Zhao, L. Effects of Flammulina velutipes polysaccharide on immune response and intestinal microbiota in mice. J. Funct. Foods 2019, 56, 255–264. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Tang, X.; Mao, B.; Zhang, Q.; Zhao, J.; Cui, S.; Chen, W. Ethanol Extract of Licorice Alleviates HFD-Induced Liver Fat Accumulation in Association with Modulation of Gut Microbiota and Intestinal Metabolites in Obesity Mice. Nutrients 2022, 14, 4180. https://doi.org/10.3390/nu14194180
Liu F, Tang X, Mao B, Zhang Q, Zhao J, Cui S, Chen W. Ethanol Extract of Licorice Alleviates HFD-Induced Liver Fat Accumulation in Association with Modulation of Gut Microbiota and Intestinal Metabolites in Obesity Mice. Nutrients. 2022; 14(19):4180. https://doi.org/10.3390/nu14194180
Chicago/Turabian StyleLiu, Fei, Xin Tang, Bingyong Mao, Qiuxiang Zhang, Jianxin Zhao, Shumao Cui, and Wei Chen. 2022. "Ethanol Extract of Licorice Alleviates HFD-Induced Liver Fat Accumulation in Association with Modulation of Gut Microbiota and Intestinal Metabolites in Obesity Mice" Nutrients 14, no. 19: 4180. https://doi.org/10.3390/nu14194180