Absorption and Tissue Distribution of Folate Forms in Rats: Indications for Specific Folate Form Supplementation during Pregnancy
Abstract
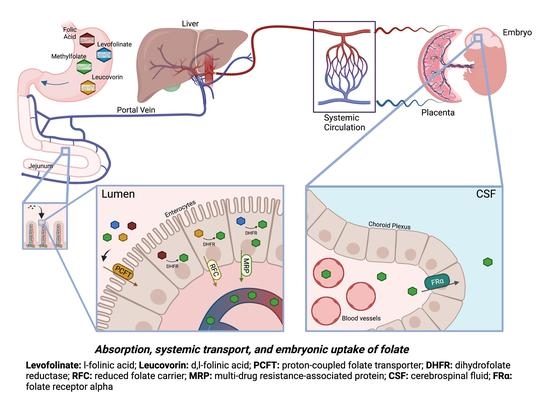
:1. Introduction
2. Materials and Methods
2.1. Folate Forms and Administration to Adult Rats
2.2. Time Course of Folate Absorption
2.3. MTHF Concentration in Tissues
2.4. Serum Folate Forms Determination by LC-ESI-MS/MS
2.5. Serum and Tissue MTHF Quantification by Sequential Binding Radio-Assay
2.6. Statistical Analysis of Tissue MTHF Values
2.7. 3H-PGA Distribution in the Presence of FRαAb
2.8. B-PGA Distribution in GD14 Placenta and Embryo
2.9. FRαAb Localization in GD14 Placenta and Embryo
3. Results
3.1. Time Course of Folate Absorption
3.2. MTHF Concentration in Non-Pregnant and Pregnant Rat Tissues Post Folate Form Administration
3.3. MTHF Concentration Post Administration of Folate Forms in the Presence of NR-IgG or FRαAb
3.4. 3H-PGA Uptake at GD14 in the Presence of FRαAb
3.5. B-PGA Distribution in GD14 Tissues
3.6. FRαAb Distribution in GD14 Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Revuelta, J.L.; Serrano-Amatriain, C.; Ledesma-Amaro, R.; Jimenez, A. Formation of folates by microorganisms: Towards the biotechnological production of this vitamin. Appl. Microbiol. Biotechnol. 2018, 102, 8613–8620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hepner, G.W.; Booth, C.C.; Cowan, J.; Hoffbrand, A.V.; Mollin, D.L. Absorption of crystalline folic acid in man. Lancet 1968, 2, 302–306. [Google Scholar] [CrossRef]
- Olinger, E.J.; Bertino, J.R.; Binder, H.J. Intestinal folate absorption. II. Conversion and retention of pteroylmonoglutamate by jejunum. J. Clin. Investig. 1973, 52, 2138–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matherly, L.H.; Goldman, D.I. Membrane transport of folates. Vitam. Horm. 2003, 66, 403–456. [Google Scholar] [CrossRef]
- Balamurugan, K.; Said, H.M. Role of reduced folate carrier in intestinal folate uptake. Am. J. Physiol. Cell Physiol. 2006, 291, C189–C193. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Matherly, L.H.; Goldman, I.D. Membrane transporters and folate homeostasis: Intestinal absorption and transport into systemic compartments and tissues. Expert Rev. Mol. Med. 2009, 11, e4. [Google Scholar] [CrossRef] [Green Version]
- Butterworth, C.E., Jr.; Baugh, C.M.; Krumdieck, C. A study of folate absorption and metabolism in man utilizing carbon-14—labeled polyglutamates synthesized by the solid phase method. J. Clin. Investig. 1969, 48, 1131–1142. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, V.M.; Pratt, R.; Viallet, A.; Cooper, B.A. Intestinal conversion of folinic acid to 5-methyltetrahydrofolate in man. Br. J. Haematol. 1972, 22, 63–72. [Google Scholar] [CrossRef]
- Visentin, M.; Diop-Bove, N.; Zhao, R.; Goldman, I.D. The intestinal absorption of folates. Annu. Rev. Physiol. 2014, 76, 251–274. [Google Scholar] [CrossRef] [Green Version]
- Hoffbrand, A.V.; Tripp, E.; Lavoie, A. Synthesis of folate polyglutamates in human cells. Clin. Sci. Mol. Med. 1976, 50, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Herbert, V. Megaloblastic anemias. Lab Investig. 1985, 52, 3–19. [Google Scholar] [PubMed]
- Green, R.; Miller, J.W. Folate deficiency beyond megaloblastic anemia: Hyperhomocysteinemia and other manifestations of dysfunctional folate status. Semin. Hematol. 1999, 36, 47–64. [Google Scholar] [PubMed]
- Forges, T.; Monnier-Barbarino, P.; Alberto, J.M.; Gueant-Rodriguez, R.M.; Daval, J.L.; Gueant, J.L. Impact of folate and homocysteine metabolism on human reproductive health. Hum. Reprod. Update 2007, 13, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Vo, H.D.; Sequeira, J.M.; Quadros, E.V.; Schwarz, S.M.; Perenyi, A.R. The role of folate receptor autoantibodies in preterm birth. Nutrition 2015, 31, 1224–1227. [Google Scholar] [CrossRef]
- Berrocal-Zaragoza, M.I.; Fernandez-Ballart, J.D.; Murphy, M.M.; Cavalle-Busquets, P.; Sequeira, J.M.; Quadros, E.V. Association between blocking folate receptor autoantibodies and subfertility. Fertil. Steril. 2009, 91, 1518–1521. [Google Scholar] [CrossRef] [Green Version]
- Rothenberg, S.P.; da Costa, M.P.; Sequeira, J.M.; Cracco, J.; Roberts, J.L.; Weedon, J.; Quadros, E.V. Autoantibodies against folate receptors in women with a pregnancy complicated by a neural-tube defect. N. Engl. J. Med. 2004, 350, 134–142. [Google Scholar] [CrossRef]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., 3rd; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development—Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef] [Green Version]
- Tam, C.; O’Connor, D.; Koren, G. Circulating unmetabolized folic Acid: Relationship to folate status and effect of supplementation. Obstet. Gynecol. Int. 2012, 2012, 485179. [Google Scholar] [CrossRef]
- Harlan De Crescenzo, A.; Panoutsopoulos, A.A.; Tat, L.; Schaaf, Z.; Racherla, S.; Henderson, L.; Leung, K.Y.; Greene, N.D.E.; Green, R.; Zarbalis, K.S. Deficient or Excess Folic Acid Supply During Pregnancy Alter Cortical Neurodevelopment in Mouse Offspring. Cereb. Cortex 2021, 31, 635–649. [Google Scholar] [CrossRef]
- Barua, S.; Kuizon, S.; Junaid, M.A. Folic acid supplementation in pregnancy and implications in health and disease. J. Biomed. Sci. 2014, 21, 77. [Google Scholar] [CrossRef] [Green Version]
- Strickland, K.C.; Krupenko, N.I.; Krupenko, S.A. Molecular mechanisms underlying the potentially adverse effects of folate. Clin. Chem. Lab. Med. 2013, 51, 607–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, R.J. Lack of historical evidence to support folic acid exacerbation of the neuropathy caused by vitamin B12 deficiency. Am. J. Clin. Nutr. 2019, 110, 554–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, E.H. Benefits and risks of folic acid to the nervous system. J. Neurol. Neurosurg. Psychiatry 2002, 72, 567–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosín-Tomás, M.; Luan, Y.; Leclerc, D.; Malysheva, O.V.; Lauzon, N.; Bahous, R.H.; Christensen, K.E.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Behavioral Alterations in Offspring with Sex-Specific Changes in Methyl Metabolism. Nutrients 2020, 12, 1716. [Google Scholar] [CrossRef]
- Ramaekers, V.T.; Quadros, E.V.; Sequeira, J.M. Role of folate receptor autoantibodies in infantile autism. Mol. Psychiatry 2013, 18, 270–271. [Google Scholar] [CrossRef] [Green Version]
- Quadros, E.V.; Sequeira, J.M.; Brown, W.T.; Mevs, C.; Marchi, E.; Flory, M.; Jenkins, E.C.; Velinov, M.T.; Cohen, I.L. Folate receptor autoantibodies are prevalent in children diagnosed with autism spectrum disorder, their normal siblings and parents. Autism Res. 2018, 11, 707–712. [Google Scholar] [CrossRef]
- Frye, R.E.; Sequeira, J.M.; Quadros, E.V.; James, S.J.; Rossignol, D.A. Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol. Psychiatry 2013, 18, 369–381. [Google Scholar] [CrossRef]
- Frye, R.E.; Delhey, L.; Slattery, J.; Tippett, M.; Wynne, R.; Rose, S.; Kahler, S.G.; Bennuri, S.C.; Melnyk, S.; Sequeira, J.M.; et al. Blocking and Binding Folate Receptor Alpha Autoantibodies Identify Novel Autism Spectrum Disorder Subgroups. Front. Neurosci. 2016, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Desai, A.; Sequeira, J.M.; Quadros, E.V. The metabolic basis for developmental disorders due to defective folate transport. Biochimie 2016, 126, 31–42. [Google Scholar] [CrossRef]
- Ramaekers, V.T.; Sequeira, J.M.; DiDuca, M.; Vrancken, G.; Thomas, A.; Philippe, C.; Peters, M.; Jadot, A.; Quadros, E.V. Improving Outcome in Infantile Autism with Folate Receptor Autoimmunity and Nutritional Derangements: A Self-Controlled Trial. Autism Res. Treat. 2019, 2019, 7486431. [Google Scholar] [CrossRef] [Green Version]
- Sequeira, J.M.; Desai, A.; Berrocal-Zaragoza, M.I.; Murphy, M.M.; Fernandez-Ballart, J.D.; Quadros, E.V. Exposure to Folate Receptor Alpha Antibodies during Gestation and Weaning Leads to Severe Behavioral Deficits in Rats: A Pilot Study. PLoS ONE 2016, 11, e0152249. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Sequeira, J.M.; Quadros, E.V. Prevention of behavioral deficits in rats exposed to folate receptor antibodies: Implication in autism. Mol. Psychiatry 2017, 22, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, S.P.; da Costa, M.; Rosenberg, Z. A radioassay for serum folate: Use of a two-phase sequential-incubation, ligand-binding system. N. Engl. J. Med. 1972, 286, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, S.P.; da Costa, M.; Lawson, J.; Rosenberg, Z. The determination of erythrocyte folate concentration using a two-phase ligand-binding radioassay. Blood 1974, 43, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, J.M.; Ramaekers, V.T.; Quadros, E.V. The diagnostic utility of folate receptor autoantibodies in blood. Clin. Chem. Lab. Med. 2013, 51, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Berrocal-Zaragoza, M.I.; Sequeira, J.; Murphy, M.M.; Fernandez-Ballart, J.D.; Abdel Baki, S.G.; Bergold, P.J.; Quadros, E.V. Folate deficiency in rat pups during weaning causes learning and memory deficits. Br. J. Nutr. 2014, 112, 1323–1332. [Google Scholar] [CrossRef] [Green Version]
- Varela-Moreiras, G.; Selhub, J. Long-term folate deficiency alters folate content and distribution differentially in rat tissues. J. Nutr. 1992, 122, 986–991. [Google Scholar] [CrossRef]
- Wolff, T.; Witkop, C.T.; Miller, T.; Syed, S.B. Preventive Services Task Force. Folic acid supplementation for the prevention of neural tube defects: An update of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2009, 150, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Grapp, M.; Just, I.A.; Linnankivi, T.; Wolf, P.; Lücke, T.; Häusler, M.; Gärtner, J.; Steinfeld, R. Molecular characterization of folate receptor 1 mutations delineates cerebral folate transport deficiency. Brain 2012, 135, 2022–2031. [Google Scholar] [CrossRef]
- Frye, R.E.; Slattery, J.C.; Quadros, E.V. Folate metabolism abnormalities in autism: Potential biomarkers. Biomark. Med. 2017, 11, 687–699. [Google Scholar] [CrossRef] [Green Version]
- Ramaekers, V.T.R.; Rothenberg, S.P.; Sequeira, J.M.; Opladen, T.; Blau, N.; Quadros, E.V.; Selhub, J. Autoantibodies to folate receptors in the cerebral folate deficiency syndrome. N. Engl. J. Med. 2005, 352, 1985–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramaekers, V.T.; Sequeira, J.M.; Quadros, E.V. The basis for folinic acid treatment in neuro-psychiatric disorders. Biochimie 2016, 126, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Weir, D.G.; Brown, J.P.; Freedman, D.S.; Scott, J.M. The absorption of the diastereoisomers of 5-methyltetrahydropteroylglutamate in man: A carrier-mediated process. Clin. Sci. Mol. Med. 1973, 45, 625–631. [Google Scholar] [CrossRef]
- Selhub, J.; Brin, H.; Grossowicz, N. Uptake and reduction of radioactive folate by everted sacs of rat small intestine. Eur. J. Biochem. 1973, 33, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.E.; Campbell, C.L.; Hillman, R.S. Kinetics of the normal folate enterohepatic cycle. J. Clin. Investig. 1979, 64, 83–88. [Google Scholar] [CrossRef]
- Fekete, K.; Berti, C.; Cetin, I.; Hermoso, M.; Koletzko, B.V.; Desci, T. Perinatal folate supply: Relevance in health outcome parameters. Matern. Child Nutr. 2010, 6, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Toriello, H. Policy and Practice Guideline Committee of the American College of Medical Genetics. Policy statement on folic acid and neural tube defects. Genet. Med. 2011, 13, 593–596. [Google Scholar] [CrossRef] [Green Version]
- Wiens, D.; DeSoto, M.C. Is High Folic Acid Intake a Risk Factor for Autism?—A Review. Brain Sci. 2017, 7, 149. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, J.A.; Bell, S.J.; Guan, Y.; Yu, Y.H. Folic Acid supplementation and pregnancy: More than just neural tube defect prevention. Rev. Obstet. Gynecol. 2011, 4, 52–59. [Google Scholar]
- Obeid, R.; Schön, C.; Pietrzik, K.; Menzel, D.; Wilhelm, M.; Smulders, Y.; Knapp, J.P.; Böhni, R. Pharmacokinetics of Sodium and Calcium Salts of [6S]-5 Methyltetrahydrofolic Acid Compared to Folic Acid and Indirect Comparison of the Two Salts. Nutrients 2020, 12, 3623. [Google Scholar] [CrossRef]
- Frye, R.E.; Slattery, J.; Delhey, L.; Furgerson, B.; Strickland, T.; Tippett, M.; Sailey, A.; Wynne, R.; Rose, S.; Melnyk, S.; et al. Folinic acid improves verbal communication in children with autism and language impairment: A randomized double-blind placebo-controlled trial. Mol. Psychiatry 2018, 23, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobrowski-Khoury, N.; Sequeira, J.M.; Arning, E.; Bottiglieri, T.; Quadros, E.V. Absorption and Tissue Distribution of Folate Forms in Rats: Indications for Specific Folate Form Supplementation during Pregnancy. Nutrients 2022, 14, 2397. https://doi.org/10.3390/nu14122397
Bobrowski-Khoury N, Sequeira JM, Arning E, Bottiglieri T, Quadros EV. Absorption and Tissue Distribution of Folate Forms in Rats: Indications for Specific Folate Form Supplementation during Pregnancy. Nutrients. 2022; 14(12):2397. https://doi.org/10.3390/nu14122397
Chicago/Turabian StyleBobrowski-Khoury, Natasha, Jeffrey M. Sequeira, Erland Arning, Teodoro Bottiglieri, and Edward V. Quadros. 2022. "Absorption and Tissue Distribution of Folate Forms in Rats: Indications for Specific Folate Form Supplementation during Pregnancy" Nutrients 14, no. 12: 2397. https://doi.org/10.3390/nu14122397