Vitamin C and Omega-3 Fatty Acid Intake Is Associated with Human Periodontitis—A Nested Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment
2.2. Phenotyping of Dental Status and Questionnaire
2.3. Matching Periodontitis Patients to Controls
2.4. Diet Acquisition
2.5. Blood Sample Preparation
2.6. Blood Sample Measurement and Data Evaluation
2.7. Statistical Analysis
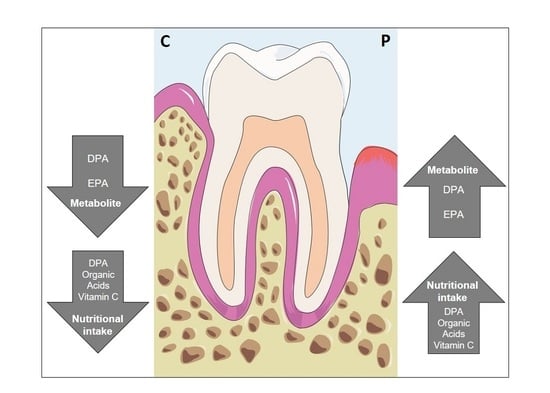
3. Results
3.1. Daily Calorie Intake Showed No Difference between Periodontal Health and Disease
3.2. Minerals, Protein, Carbohydrates and Fibers
3.3. Vitamin C Intake Is Increased in Subjects with Periodontitis
3.4. Fatty Acids
3.5. Serum Concentrations—Metabolome Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C.; Robertson, P.B. The biology, prevention, diagnosis and treatment of periodontal diseases: Scientific advances in the United States. J. Am. Dent. Assoc. 2009, 140 (Suppl. 1), 36s–43s. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontology 2013, 62, 59–94. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Blanco, J.; Buchalla, W.; Carvalho, J.C.; Dietrich, T.; Dorfer, C.; Eaton, K.A.; Figuero, E.; Frencken, J.E.; Graziani, F.; et al. Prevention and control of dental caries and periodontal diseases at individual and population level: Consensus report of group 3 of joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S85–S93. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants; Methodological Consultants. Treatment of Stage I-III Periodontitis-The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Enwonwu, C.O.; Ritchie, C.S. Nutrition and inflammatory markers. J. Am. Dent. Assoc. 2007, 138, 70–73. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, M.S.; Kim, E.J.; Ahn, Y.B.; Kim, H.D. The association of dietary vitamin C intake with periodontitis among Korean adults: Results from KNHANES. PLoS ONE 2017, 12, e0177074. [Google Scholar] [CrossRef] [Green Version]
- Amaliya; Timmerman, M.F.; Abbas, F.; Loos, B.G.; Van der Weijden, G.A.; Van Winkelhoff, A.J.; Winkel, E.G.; Van der Velden, U. Java project on periodontal diseases: The relationship between vitamin C and the severity of periodontitis. J. Clin. Periodontol. 2007, 34, 299–304. [Google Scholar] [CrossRef]
- Staudte, H.; Kranz, S.; Volpel, A.; Schutze, J.; Sigusch, B.W. Comparison of nutrient intake between patients with periodontitis and healthy subjects. Quintessence Int. 2012, 43, 907–916. [Google Scholar]
- Kuzmanova, D.; Jansen, I.D.; Schoenmaker, T.; Nazmi, K.; Teeuw, W.J.; Bizzarro, S.; Loos, B.G.; van der Velden, U. Vitamin C in plasma and leucocytes in relation to periodontitis. J. Clin. Periodontol. 2012, 39, 905–912. [Google Scholar] [CrossRef]
- Iwasaki, M.; Taylor, G.W.; Moynihan, P.; Yoshihara, A.; Muramatsu, K.; Watanabe, R.; Miyazaki, H. Dietary ratio of n-6 to n-3 polyunsaturated fatty acids and periodontal disease in community-based older Japanese: A 3-year follow-up study. Prostaglandins Leukot Essent Fat. Acids 2011, 85, 107–112. [Google Scholar] [CrossRef]
- Ramirez-Tortosa, M.C.; Quiles, J.L.; Battino, M.; Granados, S.; Morillo, J.M.; Bompadre, S.; Newman, H.N.; Bullon, P. Periodontitis is associated with altered plasma fatty acids and cardiovascular risk markers. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 133–139. [Google Scholar] [CrossRef]
- Staudte, H.; Sigusch, B.W.; Glockmann, E. Grapefruit consumption improves vitamin C status in periodontitis patients. Br. Dent. J. 2005, 199, discussion 210. [Google Scholar] [CrossRef]
- Dodington, D.W.; Fritz, P.C.; Sullivan, P.J.; Ward, W.E. Higher Intakes of Fruits and Vegetables, beta-Carotene, Vitamin C, alpha-Tocopherol, EPA, and DHA Are Positively Associated with Periodontal Healing after Nonsurgical Periodontal Therapy in Nonsmokers but Not in Smokers. J. Nutr. 2015, 145, 2512–2519. [Google Scholar] [CrossRef] [Green Version]
- Muniz, F.W.; Nogueira, S.B.; Mendes, F.L.; Rosing, C.K.; Moreira, M.M.; de Andrade, G.M.; Carvalho Rde, S. The impact of antioxidant agents complimentary to periodontal therapy on oxidative stress and periodontal outcomes: A systematic review. Arch. Oral. Biol. 2015, 60, 1203–1214. [Google Scholar] [CrossRef]
- Woelber, J.P.; Bremer, K.; Vach, K.; Konig, D.; Hellwig, E.; Ratka-Kruger, P.; Al-Ahmad, A.; Tennert, C. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—A randomized controlled pilot study. BMC Oral Health 2016, 17, 28. [Google Scholar] [CrossRef] [Green Version]
- Van der Velden, U.; Kuzmanova, D.; Chapple, I.L. Micronutritional approaches to periodontal therapy. J. Clin. Periodontol. 2011, 38 (Suppl. 11), 142–158. [Google Scholar] [CrossRef]
- Chee, B.; Park, B.; Fitzsimmons, T.; Coates, A.M.; Bartold, P.M. Omega-3 fatty acids as an adjunct for periodontal therapy-a review. Clin. Oral. Investig. 2016, 20, 879–894. [Google Scholar] [CrossRef]
- Chapple, I.L.; Milward, M.R.; Ling-Mountford, N.; Weston, P.; Carter, K.; Askey, K.; Dallal, G.E.; De Spirt, S.; Sies, H.; Patel, D.; et al. Adjunctive daily supplementation with encapsulated fruit, vegetable and berry juice powder concentrates and clinical periodontal outcomes: A double-blind RCT. J. Clin. Periodontol. 2012, 39, 62–72. [Google Scholar] [CrossRef]
- Schulz, J.; Knappe, C.; Graetz, C.; Mewes, L.; Türk, K.; Black, A.K.; Lieb, W.; Schäfer, A.S.; Fawzy El-Sayed, K.M.; Dörfer, C.E.; et al. Secreted frizzled-related protein 5 serum levels in human periodontitis-A nested case-control study. J. Clin. Periodontol. 2019, 46, 522–528. [Google Scholar] [CrossRef]
- Muller, N.; Schulte, D.M.; Turk, K.; Freitag-Wolf, S.; Hampe, J.; Zeuner, R.; Schroder, J.O.; Gouni-Berthold, I.; Berthold, H.K.; Krone, W.; et al. IL-6 blockade by monoclonal antibodies inhibits apolipoprotein (a) expression and lipoprotein (a) synthesis in humans. J. Lipid Res. 2015, 56, 1034–1042. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Thingholm, L.B.; Skieceviciene, J.; Rausch, P.; Kummen, M.; Hov, J.R.; Degenhardt, F.; Heinsen, F.A.; Ruhlemann, M.C.; Szymczak, S.; et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 2016, 48, 1396–1406. [Google Scholar] [CrossRef]
- Bohlscheid-Thomas, S.; Hoting, I.; Boeing, H.; Wahrendorf, J. Reproducibility and relative validity of energy and macronutrient intake of a food frequency questionnaire developed for the German part of the EPIC project. European Prospective Investigation into Cancer and Nutrition. Int. J. Epidemiol. 1997, 26 (Suppl. 1), S71–S81. [Google Scholar] [CrossRef] [Green Version]
- Boeing, H.; Bohlscheid-Thomas, S.; Voss, S.; Schneeweiss, S.; Wahrendorf, J. The relative validity of vitamin intakes derived from a food frequency questionnaire compared to 24-hour recalls and biological measurements: Results from the EPIC pilot study in Germany. European Prospective Investigation into Cancer and Nutrition. Int. J. Epidemiol. 1997, 26, S82–S90. [Google Scholar] [CrossRef] [Green Version]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Kind, T.; Fiehn, O. Seven Golden Rules for heuristic filtering of molecular formulas obtained by accurate mass spectrometry. BMC Bioinform. 2007, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- R Foundation for Statistical Computin. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Tonetti, M.S.; Van Dyke, T.E.; Working Group 1 of the Joint EFP/AAP Workshop. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S24–S29. [Google Scholar] [CrossRef] [PubMed]
- Mieko, N.; Grossi, G.S.; Dunford, D.R.; Ho, A.W.; Trevisan, M.; Genco, G.R. Dietary Vitamin C and the Risk for Periodontal Disease. J. Periodontol. 2000, 71, 1215–1223. [Google Scholar] [CrossRef]
- Enwonwu, C.O.; Phillips, R.S.; Falkler, W.A., Jr. Nutrition and oral infectious diseases: State of the science. Compend. Contin. Educ. Dent. 2002, 23, 431–434, 436, 438 passim; quiz 448. [Google Scholar] [PubMed]
- De Jong, T.M.; Jochens, A.; Jockel-Schneider, Y.; Harks, I.; Dommisch, H.; Graetz, C.; Flachsbart, F.; Staufenbiel, I.; Eberhard, J.; Folwaczny, M.; et al. SLC23A1 polymorphism rs6596473 in the vitamin C transporter SVCT1 is associated with aggressive periodontitis. J. Clin. Periodontol. 2014, 41, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Van Woudenbergh, G.J.; Theofylaktopoulou, D.; Kuijsten, A.; Ferreira, I.; van Greevenbroek, M.M.; van der Kallen, C.J.; Schalkwijk, C.G.; Stehouwer, C.D.; Ocke, M.C.; Nijpels, G.; et al. Adapted dietary inflammatory index and its association with a summary score for low-grade inflammation and markers of glucose metabolism: The Cohort study on Diabetes and Atherosclerosis Maastricht (CODAM) and the Hoorn study. Am. J. Clin. Nutr. 2013, 98, 1533–1542. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, A.Z.; Buettner, C.; Phillips, R.S.; Davis, R.B.; Mukamal, K.J. n-3 fatty acids and periodontitis in US adults. J. Am. Diet. Assoc. 2010, 110, 1669–1675. [Google Scholar] [CrossRef] [Green Version]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef] [Green Version]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef] [Green Version]



| Periodontitis Patients | Controls | p-Value | |
|---|---|---|---|
| All periodontitis patients (unstratified) and controls | |||
| Subjects (n) | 326 | 326 | - |
| Male (%) | 40 | 40 | p = 1 |
| Smoker (%) | 65 | 65 | p = 1 |
| Age (yrs) | 59 (50–67) | 59 (50–67) | p = 0.784 |
| BMI (kg/m2) | 28.7 (24.9–36.9) | 27.5 (24.4–35.5) | p = 0.087 |
| Periodontitis without tooth loss and controls | |||
| Subjects (n) | 213 | 213 | - |
| Male (%) | 38 | 38 | p = 1 |
| Smoker (%) | 63 | 63 | p = 1 |
| Age (yrs) | 56 (48–65) | 57 (49–65) | p = 0.738 |
| BMI (kg/m2) | 28 (24.7–36.8) | 27.5 (24.3–33.8) | p = 0.248 |
| Periodontitis with tooth loss and controls | |||
| Subjects (n) | 113 | 113 | - |
| Male (%) | 46 | 46 | p = 1 |
| Smoker (%) | 75 | 75 | p = 1 |
| Age (yrs) | 63 (55–69) | 63 (53–70) | p = 0.933 |
| BMI (kg/m2) | 29.9 (25.5–37.8) | 27.5 (25.1–35.8) | p = 0.158 |
| Component (C vs. P) | p-Value | Component (C vs. P) | p-Value | ||
|---|---|---|---|---|---|
| fatty acids | All saturated fatty acids | p = 0.086 | vitamins | vitamins | p = 0.020 |
| Octadecanoic acid | p = 0.057 | fat | p = 0.058 | ||
| Octanoic acid | p = 0.080 | proteins | p = 0.077 | ||
| Hexadecanoic acid | p = 0.080 | minerals | p = 0.131 | ||
| Dodecanoic acid | p = 0.082 | carbohydrates | p = 0.255 | ||
| Eicosanoic acid | p = 0.105 | fibers | p = 0.342 | ||
| Tetradecanoic acid | p = 0.144 | vitamin C | p = 0.007 | ||
| Decanoic acid | p = 0.154 | vitamin D | p = 0.033 | ||
| Butanoic acid | p = 0.292 | vitamin E | p = 0.064 | ||
| Heptadecanoic acid | p = 0.291 | vitamin B5 | p = 0.080 | ||
| Hexanoic acid | p = 0.294 | vitamin B1 | p = 0.085 | ||
| Pentadecanoic acid | p = 0.331 | vitamin B6 | p = 0.088 | ||
| Teracosanoic acid | p = 0.342 | vitamin B9 | p = 0.148 | ||
| Decosanoic acid | p = 0.393 | vitamin B3 | p = 0.152 | ||
| All monounsaturated fatty acids | p = 0.057 | vitamin B7 | p = 0.197 | ||
| Eicosenoic acid | p = 0.009 | vitamin K | p = 0.199 | ||
| Decosenoic acid | p = 0.011 | vitamin B12 | p = 0.232 | ||
| Tetracosenic acid | p = 0.015 | vitamin B2 | p = 0.326 | ||
| Hexadecenoic acid | p = 0.053 | vitamin A | p = 0.841 | ||
| Octadecenoic acid | p = 0.061 | ||||
| Heptadecenoic acid | p = 0.325 | ||||
| Tetradecenoic acid | p = 0.342 | ||||
| Pentadecenoic acid | p = 0.344 | ||||
| All polyunsaturated fatty acids | p = 0.087 | ||||
| Docosapentaenoic acid | p = 0.003 * | ||||
| Docosahexaenoic acid | p = 0.008 | ||||
| Eicodonic acid | p = 0.018 | ||||
| Eicosatrienoic acid | p = 0.018 | ||||
| Eicosatetraenoic acid | p = 0.019 | ||||
| Octadecatetraenoic acid | p = 0.024 | ||||
| Hexadecadienoic acid | p = 0.025 | ||||
| Docosatetraenoic acid | p = 0.037 | ||||
| Eicosadienoic acid | p = 0.043 | ||||
| Octadecatrienoic acid | p = 0.083 | ||||
| Octadecadienoic acid | p = 0.13 | ||||
| Nonadecatrienoic acid | p = 0.243 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mewes, L.; Knappe, C.; Graetz, C.; Wagner, J.; Demetrowitsch, T.J.; Jensen-Kroll, J.; Mohamed Fawzy El-Sayed, K.; Schwarz, K.; Dörfer, C.E.; Schreiber, S.; et al. Vitamin C and Omega-3 Fatty Acid Intake Is Associated with Human Periodontitis—A Nested Case-Control Study. Nutrients 2022, 14, 1939. https://doi.org/10.3390/nu14091939
Mewes L, Knappe C, Graetz C, Wagner J, Demetrowitsch TJ, Jensen-Kroll J, Mohamed Fawzy El-Sayed K, Schwarz K, Dörfer CE, Schreiber S, et al. Vitamin C and Omega-3 Fatty Acid Intake Is Associated with Human Periodontitis—A Nested Case-Control Study. Nutrients. 2022; 14(9):1939. https://doi.org/10.3390/nu14091939
Chicago/Turabian StyleMewes, Louisa, Carina Knappe, Christian Graetz, Juliane Wagner, Tobias J. Demetrowitsch, Julia Jensen-Kroll, Karim Mohamed Fawzy El-Sayed, Karin Schwarz, Christof E. Dörfer, Stefan Schreiber, and et al. 2022. "Vitamin C and Omega-3 Fatty Acid Intake Is Associated with Human Periodontitis—A Nested Case-Control Study" Nutrients 14, no. 9: 1939. https://doi.org/10.3390/nu14091939