Caloric Restriction per se Rather Than Dietary Macronutrient Distribution Plays a Primary Role in Metabolic Health and Body Composition Improvements in Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
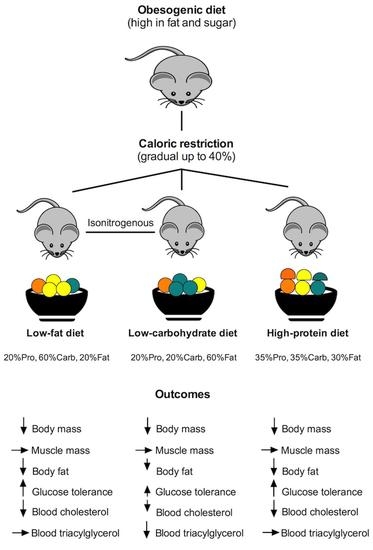
2.1. Animals and Experimental Design
2.2. Caloric Restriction
2.3. Glucose Tolerance
2.4. Energy Metabolism and Physical Activity
2.5. Blood Lipids
2.6. Body Composition
2.7. Statistical Analysis
3. Results
3.1. All Diets Had Similar Effect on Body Composition in Obese Mice
3.2. Blood Glucose and Cholesterol Improved with Weight Loss
3.3. Energy Intake, Energy Metabolism and Activity Were Similar between the Diet Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Global BMI Mortality Collaboration; Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; de Gonzalez, A.B.; Cairns, B.; Huxley, R.; Jackson, C.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef] [Green Version]
- National Task Force on the Prevention and Treatment of Obesity; Billington, C.J.; Epstein, L.H.; Goodwin, N.J.; Hill, J.O.; Pi-Sunyer, F.X.; Rolls, B.J.; Stern, J.; Wadden, T.A.; Weinsier, R.L.; et al. Overweight, obesity, and health risk. Arch. Intern. Med. 2000, 160, 898–904. [Google Scholar] [CrossRef]
- Westerterp, K.R. Exercise for weight loss. Am. J. Clin. Nutr. 2019, 110, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, A.C.; Schoeller, D.A. Is a calorie a calorie? Am. J. Clin. Nutr. 2004, 79, 899S–906S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leaf, A.; Antonio, J. The effects of overfeeding on body composition: The role of macronutrient composition—A narrative review. Int. J. Exerc. Sci. 2017, 10, 1275–1296. [Google Scholar] [PubMed]
- Weigle, D.S.; Breen, P.A.; Matthys, C.C.; Callahan, H.S.; Meeuws, K.E.; Burden, V.R.; Purnell, J.Q. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am. J. Clin. Nutr. 2005, 82, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R. Diet induced thermogenesis. Nutr. Metab. 2004, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Westerterp-Plantenga, M.S.; Lejeune, M.P.G.M.; Nijs, I.; Van Ooijen, M.; Kovacs, E.M.R. High protein intake sustains weight maintenance after body weight loss in humans. Int. J. Obes. Relat. Metab. Disord. 2003, 28, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Drummen, M.; Tischmann, L.; Gatta-Cherifi, B.; Fogelholm, M.; Raben, A.; Adam, T.C.; Westerterp-Plantenga, M.S. High compared with moderate protein intake reduces adaptive thermogenesis and induces a negative energy balance during long-term weight-loss maintenance in participants with prediabetes in the postobese state: A preview study. J. Nutr. 2019, 150, 458–463. [Google Scholar] [CrossRef]
- Hall, K.D. The potential role of protein leverage in the US obesity epidemic. Obesity 2019, 27, 1222–1224. [Google Scholar] [CrossRef] [Green Version]
- Simpson, S.J.; Raubenheimer, D. Obesity: The protein leverage hypothesis. Obes. Rev. 2005, 6, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, A.; Mayntz, D.; Raubenheimer, D.; Simpson, S.J. Protein-leverage in mice: The geometry of macronutrient balancing and consequences for fat deposition. Obesity 2008, 16, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Blair, A.R.; Strube, M.L.; Proietto, J.; Andrikopoulos, S. Improving glucose tolerance by reducing weight gain in a polygenic obese mouse model: Use of a high protein diet. Horm. Metab. Res. 2014, 47, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Ebbeling, C.B. The Carbohydrate-Insulin Model of Obesity: Beyond “Calories In, Calories Out”. JAMA Intern. Med. 2018, 178, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Guyenet, S.J.; Leibel, R.L. The carbohydrate-insulin model of obesity is difficult to reconcile with current evidence. JAMA Intern. Med. 2018, 178, 1103–1105. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. The challenge of reforming nutritional epidemiologic research. JAMA 2018, 320, 969–970. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D. Challenges of human nutrition research. Science 2020, 367, 1298–1300. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef] [Green Version]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019, 43, 1491–1492. [Google Scholar] [CrossRef] [Green Version]
- Minderis, P.; Fokin, A.; Dirmontas, M.; Ratkevicius, A. Hypocaloric low-carbohydrate and low-fat diets with fixed protein lead to similar health outcomes in obese mice. Obesity 2020, 28, 1494–1502. [Google Scholar] [CrossRef]
- Fokin, A.; Minderis, P.; Venckunas, T.; Lionikas, A.; Kvedaras, M.; Ratkevicius, A. Myostatin dysfunction does not protect from fasting-induced loss of muscle mass in mice. J. Musculoskelet. Neuronal Interact. 2019, 19, 342–353. [Google Scholar] [PubMed]
- Oldknow, K.; Macrae, V.; Farquharson, C.; Bunger, L. Evaluating invasive and non-invasive methods to determine fat content in the laboratory mouse. Open Life Sci. 2015, 10, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Tschoep, M.; Speakman, J.R.; Arch, J.R.S.; Auwerx, J.; Brüning, J.C.; Chan, L.; Eckel, R.H.; Farese, R.V.; Galgani, J.E.; Hambly, C.; et al. A guide to analysis of mouse energy metabolism. Nat. Methods 2011, 9, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Wang, L.; Togo, J.; Yang, D.; Xu, Y.; Wu, Y.; Douglas, A.; Speakman, J.R. The carbohydrate-insulin model does not explain the impact of varying dietary macronutrients on the body weight and adiposity of mice. Mol. Metab. 2020, 32, 27–43. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.; Yang, D.; Li, L.; Togo, J.; Wu, Y.; Liu, Q.; Li, B.; Li, M.; Wang, G.; et al. Dietary fat, but not protein or carbohydrate, regulates energy intake and causes adiposity in mice. Cell Metab. 2018, 28, 415–431.e4. [Google Scholar] [CrossRef] [Green Version]
- Solon-Biet, S.; McMahon, A.C.; Ballard, J.W.O.; Ruohonen, K.; Wu, L.E.; Cogger, V.C.; Warren, A.; Huang, X.; Pichaud, N.; Melvin, R.; et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014, 19, 418–430. [Google Scholar] [CrossRef] [Green Version]
- Ebbeling, C.B.; Feldman, H.A.; Klein, G.L.; Wong, J.M.W.; Bielak, L.; Steltz, S.K.; Luoto, P.K.; Wolfe, R.R.; Wong, W.W.; Ludwig, D.S. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: Randomized trial. BMJ 2018, 363, k4583. [Google Scholar] [CrossRef] [Green Version]
- Hall, K.D.; Guo, J.; Chen, K.Y.; Leibel, R.L.; Reitman, M.L.; Rosenbaum, M.; Smith, S.R.; Ravussin, E. Methodologic considerations for measuring energy expenditure differences between diets varying in carbohydrate using the doubly labeled water method. Am. J. Clin. Nutr. 2019, 109, 1328–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, K.D.; Guo, J.; Speakman, J.R. Do low-carbohydrate diets increase energy expenditure? Int. J. Obes. 2019, 43, 2350–2354. [Google Scholar] [CrossRef] [Green Version]
- Hall, K.D. Mystery or method? Evaluating claims of increased energy expenditure during a ketogenic diet. PLoS ONE 2019, 14, e0225944. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Guo, J. Obesity energetics: Body weight regulation and the effects of diet composition. Gastroenterology 2017, 152, 1718–1727.e3. [Google Scholar] [CrossRef] [Green Version]
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.; Desai, M.; King, A.C. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: The dietfits randomized clinical trial. JAMA 2018, 319, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.; Bonomi, A.G.; Lemmens, S.G.; Scholte, J.; Thijssen, M.A.; van Berkum, F.; Westerterp-Plantenga, M.S. Relatively high-protein or ‘low-carb’ energy-restricted diets for body weight loss and body weight maintenance? Physiol. Behav. 2012, 107, 374–380. [Google Scholar] [CrossRef]
- Wycherley, T.P.; Moran, L.J.; Clifton, P.M.; Noakes, M.; Brinkworth, G.D. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 1281–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasiakos, S.M.; Cao, J.J.; Margolis, L.M.; Sauter, E.R.; Whigham, L.D.; McClung, J.P.; Rood, J.C.; Carbone, J.W.; Combs, G.F., Jr.; Young, A.J. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: A randomized controlled trial. FASEB J. 2013, 27, 3837–3847. [Google Scholar] [CrossRef] [Green Version]
- Wycherley, T.; Noakes, M.; Clifton, P.M.; Cleanthous, X.; Keogh, J.B.; Brinkworth, G.D. A High-protein diet with resistance exercise training improves weight loss and body composition in overweight and obese patients with type 2 diabetes. Diabetes Care 2010, 33, 969–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.J.; Enderle, J.; Bosy-Westphal, A. Changes in energy expenditure with weight gain and weight loss in humans. Curr. Obes. Rep. 2016, 5, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, S.E.; Tang, Z.; Kerbois, C.; Delville, C.; Konstantopedos, P.; Bruel, A.; Derous, D.; Green, C.; Aspden, R.; Goodyear, S.R.; et al. The effects of graded levels of calorie restriction: I. Impact of short term calorie and protein restriction on body composition in the C57BL/6 mouse. Oncotarget 2015, 6, 15902–15930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axen, K.V.; Axen, K. Very low-carbohydrate versus isocaloric high-carbohydrate diet in dietary obese rats. Obesity 2006, 14, 1344–1352. [Google Scholar] [CrossRef]
- Bielohuby, M.; Sisley, S.; Sandoval, D.; Herbach, N.; Zengin, A.; Fischereder, M.; Menhofer, D.; Stoehr, B.J.M.; Stemmer, K.; Wanke, R.; et al. Impaired glucose tolerance in rats fed low-carbohydrate, high-fat diets. Am. J. Physiol. Metab. 2013, 305, E1059–E1070. [Google Scholar] [CrossRef] [Green Version]
- Ellenbroek, J.H.; Van Dijck, L.; Töns, H.A.; Rabelink, T.J.; Carlotti, F.; Ballieux, B.E.P.B.; De Koning, E.J.P. Long-term ketogenic diet causes glucose intolerance and reduced β- and α-cell mass but no weight loss in mice. Am. J. Physiol. Metab. 2014, 306, E552–E558. [Google Scholar] [CrossRef] [Green Version]
- Lamont, B.J.; Waters, M.F.; Andrikopoulos, S. A low-carbohydrate high-fat diet increases weight gain and does not improve glucose tolerance, insulin secretion or β-cell mass in NZO mice. Nutr. Diabetes 2016, 6, e194. [Google Scholar] [CrossRef]
- Grandl, G.; Straub, L.; Rudigier, C.; Arnold, M.; Wueest, S.; Konrad, D.; Wolfrum, C. Short-term feeding of a ketogenic diet induces more severe hepatic insulin resistance than an obesogenic high-fat diet. J. Physiol. 2018, 596, 4597–4609. [Google Scholar] [CrossRef] [PubMed]
- Vangoitsenhoven, R.; Van Der Ende, M.; Corbeels, K.; Cunha, J.P.M.C.M.; Lannoo, M.; Bedossa, P.; Van der Merwe, S.; Mertens, A.; Gesquiere, I.; Meulemans, A.; et al. At similar weight loss, dietary composition determines the degree of glycemic improvement in diet-induced obese C57BL/6 mice. PLoS ONE 2018, 13, e0200779. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.C.; Van Boom, K.M.; Armino, N.; Larmuth, K.; Noakes, T.D.; Smith, J.A.; Kohn, T.A. Reduced glucose tolerance and skeletal muscle GLUT4 and IRS1 content in cyclists habituated to a long-term low-carbohydrate, high-fat diet. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 210–217. [Google Scholar] [CrossRef]
- Shimizu, K.; Saito, H.; Sumi, K.; Sakamoto, Y.; Tachi, Y.; Iida, K. Short-term and long-term ketogenic diet therapy and the addition of exercise have differential impacts on metabolic gene expression in the mouse energy-consuming organs heart and skeletal muscle. Nutr. Res. 2018, 60, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Duivenvoorde, L.P.M.; Van Schothorst, E.M.; Bunschoten, A.; Keijer, J. Dietary restriction of mice on a high-fat diet induces substrate efficiency and improves metabolic health. J. Mol. Endocrinol. 2011, 47, 81–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matyi, S.; Jackson, J.; Garrett, K.; Deepa, S.S.; Unnikrishnan, A. The effect of different levels of dietary restriction on glucose homeostasis and metabolic memory. GeroScience 2018, 40, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Dommerholt, M.B.; Dionne, D.A.; Hutchinson, D.F.; Kruit, J.; Johnson, J.D. Metabolic effects of short-term caloric restriction in mice with reduced insulin gene dosage. J. Endocrinol. 2018, 237, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Bueno, N.B.; De Melo, I.S.V.; De Oliveira, S.L.; da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef] [Green Version]
- Ruth, M.R.; Port, A.; Shah, M.; Bourland, A.C.; Istfan, N.W.; Nelson, K.P.; Gokce, N.; Apovian, C.M. Consuming a hypocaloric high fat low carbohydrate diet for 12 weeks lowers C-reactive protein and raises serum adiponectin and high density lipoprotein-cholesterol in obese subjects. Metabolism 2013, 62, 1779–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinn, C.; McPhee, J.; Harris, N.K.; Williden, M.; Prendergast, K.; Schofield, G. A 12-week low-carbohydrate, high-fat diet improves metabolic health outcomes over a control diet in a randomised controlled trial with overweight defence force personnel. Appl. Physiol. Nutr. Metab. 2017, 42, 1158–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welty, F.K. How do elevated triglycerides and low HDL-cholesterol affect inflammation and atherothrombosis? Curr. Cardiol. Rep. 2013, 15, 400. [Google Scholar] [CrossRef] [Green Version]
- Quispe, R.; Manalac, R.J.; Faridi, K.F.; Blaha, M.J.; Toth, P.P.; Kulkarni, K.R.; Nasir, K.; Virani, S.; Banach, M.; Blumenthal, R.S.; et al. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: The Very Large Database of Lipids-4 (VLDL-4) study. Atherosclerosis 2015, 242, 243–250. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.; Raggi, P. The ketogenic diet: Pros and cons. Atherosclerosis 2020, 292, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, H.; Raynes, R.; Longo, V.D. The conserved role of protein restriction in aging and disease. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 74–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, N.E.; Konon, E.N.; Schuster, H.S.; Mitchell, A.T.; Boyle, C.; Rodgers, A.C.; Finke, M.; Haider, L.R.; Yu, D.; Flores, V.; et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and life span in mice. Nat. Aging 2021, 1, 73–86. [Google Scholar] [CrossRef]
- Speakman, J.; Mitchell, S.; Mazidi, M. Calories or protein? The effect of dietary restriction on lifespan in rodents is explained by calories alone. Exp. Gerontol. 2016, 86, 28–38. [Google Scholar] [CrossRef]



| Control | LFD | LCD | HPD | |||||
|---|---|---|---|---|---|---|---|---|
| Energy status | Ad libitum | CR up to 40% | CR up to 40% | CR up to 40% | ||||
| Diet’s manufacturer | Research Diets Inc. | Research Diets Inc. | Research Diets Inc. | Research Diets Inc. | ||||
| Diet’s number | D12451 | D17100401 | D12492 | D17100402 | ||||
| Fat (kcal%) | 45 | 20 | 60 | 30 | ||||
| Carbohydrate (kcal%) | 35 | 60 | 20 | 35 | ||||
| Protein (kcal%) | 20 | 20 | 20 | 35 | ||||
| Ingredients (in g and kcal) | ||||||||
| Protein: | ||||||||
| Casein | 23.3 | 93.3 | 20.0 | 80.1 | 25.8 | 103.3 | 38.2 | 152.6 |
| L-cystine | 0.35 | 1.4 | 0.30 | 1.2 | 0.35 | 1.4 | 0.53 | 2.1 |
| Carbohydrate: | ||||||||
| Corn starch | 8.5 | 33.9 | 40.5 | 162.1 | 0 | 0 | 16.1 | 64.4 |
| Maltodextrin | 11.7 | 46.6 | 12.5 | 50.0 | 16.1 | 64.6 | 15.9 | 63.6 |
| Sucrose | 20.1 | 80.6 | 6.9 | 27.5 | 8.9 | 35.5 | 7.3 | 29.1 |
| Fibre: | ||||||||
| Cellulose | 5.83 | 0 | 5.0 | 0 | 6.5 | 0 | 5.3 | 0 |
| Fat: | ||||||||
| Soybean oil | 2.9 | 26.2 | 2.5 | 22.5 | 3.2 | 29.1 | 2.65 | 23.8 |
| Lard | 20.7 | 186.3 | 6.5 | 58.5 | 31.6 | 284.8 | 11.66 | 104.9 |
| Minerals: | ||||||||
| Mineral mix S10026 | 1.17 | 0 | 1.00 | 0 | 1.29 | 0 | 1.06 | 0 |
| DiCalcium phosphate | 1.52 | 0 | 1.30 | 0 | 1.68 | 0 | 1.38 | 0 |
| Calcium carbonate | 0.64 | 0 | 0.55 | 0 | 0.71 | 0 | 0.58 | 0 |
| Potassium citrate | 1.92 | 0 | 1.65 | 0 | 2.13 | 0 | 1.75 | 0 |
| Vitamins: | ||||||||
| Vitamin mix V10001 | 1.17 | 4.7 | 1.00 | 4.0 | 1.29 | 5.2 | 1.06 | 4.2 |
| Choline bitartrate | 0.23 | 0 | 0.20 | 0 | 0.26 | 0 | 0.21 | 0 |
| Total | 100 | 473 | 100 | 406 | 100 | 524 | 100 | 430 |
| Kcal/g | 4.7 | 4.1 | 5.2 | 4.3 | ||||
| Control (n = 10) | LFD (n = 16) | LCD (n = 16) | HPD (n = 16) | |
|---|---|---|---|---|
| Hindlimb muscles: (in miligrams) | ||||
| Gastrocnemius | 135.8 ± 3.2 | 125.4 ± 12.8 | 129.2 ± 8.4 | 121.6 ± 18.0 # |
| Plantaris | 17.7 ± 0.6 | 16.4 ± 1.9 | 16.9 ± 1.3 | 16.2 ± 2.2 |
| Soleus | 9.7 ± 0.6 | 8.8 ± 0.8 | 9.1 ± 0.9 | 9.0 ± 1.1 |
| Tibialis anterior | 47.9 ± 2.9 | 48.2 ± 3.3 | 47.9 ± 2.8 | 47.2 ± 4.9 |
| EDL | 11.3 ± 0.6 | 10.4 ± 1.2 | 10.6 ± 0.6 | 10.1 ± 1.4 # |
| Major fat sites: (in miligrams) | ||||
| Subcutaneous | 3286.6 ± 405.4 | 880.1 ± 591.8 ### | 1595.1 ± 1210.0 # | 1036.7 ± 894.8 ### |
| Gonadal | 2275.3 ± 303.1 | 549.1 ± 364.9 ### | 1047.7 ± 714.2 ### | 617.4 ± 547.4 ### |
| Mesenteric | 1152.7 ± 207.0 | 357.6 ± 162.5 ### | 511.8 ± 349.6 ## | 381.6 ± 207.2 ### |
| Perirenal | 1007.3 ± 166.5 | 174.5 ± 147.9 ### | 348.6 ± 314.1 ## | 207.1 ± 203.1 ### |
| Intrascapular brown | 159.4 ± 31.2 | 110.7 ± 24.0 ### | 107.8 ± 27.8 ### | 106.1 ± 28.6 ### |
| Organs: (in miligrams) | ||||
| Liver | 1330.0 ± 304.0 | 848.5 ± 249.5 ### | 847.2 ± 171.8 ### | 876.2 ± 208.2 ### |
| Heart | 142.7 ± 10.1 | 130.3 ± 7.5 ## | 134.1 ± 9.2 | 131.8 ± 8.9 # |
| (% of body mass) | ||||
| Liver | 3.22 ± 0.61 | 3.03 ± 0.50 | 2.76 ± 0.20 # | 3.14 ± 0.25 |
| Heart | 0.35 ± 0.02 | 0.48 ± 0.06 ### | 0.45 ± 0.08 ## | 0.49 ± 0.09 ### |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minderis, P.; Fokin, A.; Dirmontas, M.; Kvedaras, M.; Ratkevicius, A. Caloric Restriction per se Rather Than Dietary Macronutrient Distribution Plays a Primary Role in Metabolic Health and Body Composition Improvements in Obese Mice. Nutrients 2021, 13, 3004. https://doi.org/10.3390/nu13093004
Minderis P, Fokin A, Dirmontas M, Kvedaras M, Ratkevicius A. Caloric Restriction per se Rather Than Dietary Macronutrient Distribution Plays a Primary Role in Metabolic Health and Body Composition Improvements in Obese Mice. Nutrients. 2021; 13(9):3004. https://doi.org/10.3390/nu13093004
Chicago/Turabian StyleMinderis, Petras, Andrej Fokin, Mantas Dirmontas, Mindaugas Kvedaras, and Aivaras Ratkevicius. 2021. "Caloric Restriction per se Rather Than Dietary Macronutrient Distribution Plays a Primary Role in Metabolic Health and Body Composition Improvements in Obese Mice" Nutrients 13, no. 9: 3004. https://doi.org/10.3390/nu13093004