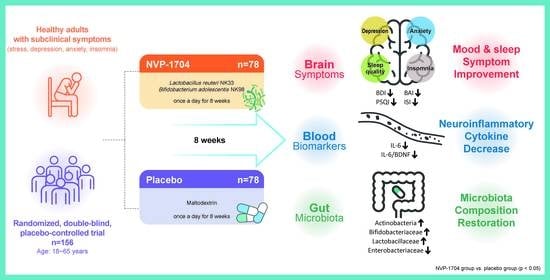
Effects of Probiotic NVP-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Intervention
2.3. Questionnaires
2.4. Blood Biomarkers
2.5. Gut Microbiome Analysis
2.6. Safety Assessment
2.7. Nutritional Data
2.8. Statistical Analysis
3. Results
3.1. Eligibility and Baseline Characteristics
3.2. Effects of NVP-1704 on Mood
3.3. Effects of NVP-1704 on Sleep
3.4. Blood Biomarkers
3.5. Gut Microbiome Analysis
3.6. Safety and Nutritional Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat-The Relationship between Diet, Microbiota, and Metabolic Disorders-A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tetel, M.J.; de Vries, G.J.; Melcangi, R.C.; Panzica, G.; O’Mahony, S.M. Steroids, stress and the gut microbiome-brain axis. J. Neuroendocrinol. 2018, 30, e12548. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galland, L. The gut microbiome and the brain. J. Med. Food 2014, 17, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.; Hart, R.A.; Charlesworth, R.P.G.; Sharpley, C.F. Gut microbiome and depression: What we know and what we need to know. Rev. Neurosci. 2018, 29, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Cresci, G.A.; Bawden, E. Gut Microbiome: What We Do and Don’t Know. Nutr. Clin. Pract. 2015, 30, 734–746. [Google Scholar] [CrossRef] [Green Version]
- Sanders, M.E. Probiotics: Definition, sources, selection, and uses. Clin. Infect. Dis. 2008, 46, S58–S61. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.H.; Liu, Y.W.; Wu, C.C.; Wang, S.; Tsai, Y.C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, A.A.; Jazayeri, S.; Khosravi-Darani, K.; Solati, Z.; Mohammadpour, N.; Asemi, Z.; Adab, Z.; Djalali, M.; Tehrani-Doost, M.; Hosseini, M.; et al. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 2016, 19, 387–395. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 2017, 51, 810–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cammarota, G.; Ianiro, G.; Bibbo, S.; Gasbarrini, A. Gut microbiota modulation: Probiotics, antibiotics or fecal microbiota transplantation? Intern. Emerg. Med. 2014, 9, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Lee, K.E.; Kim, D.H. The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice. Nutrients 2019, 11, 819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.K.; Kim, J.K.; Joo, M.K.; Lee, K.E.; Han, S.W.; Kim, D.H. Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 Alleviate Escherichia coli-Induced depression and Gut Dysbiosis in Mice. J. Microbiol. Biotechnol. 2020, 30, 1222–1226. [Google Scholar] [CrossRef]
- Koh, K.B.; Park, J.K.; Kim, C.H. Development of the stress response inventory. J. Korean Neuropsychiatr. Assoc. 2000, 39, 707–719. [Google Scholar]
- Park, K.; Jaekal, E.; Yoon, S.; Lee, S.H.; Choi, K.H. Diagnostic Utility and Psychometric Properties of the Beck Depression Inventory-II Among Korean Adults. Front Psychol. 2019, 10, 2934. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-K.; Lee, E.-H.; Hwang, S.-T.; Hong, S.-H.; Kim, J.-H. Psychometric properties of the Beck Anxiety Inventory in the community-dwelling sample of Korean adults. Korean J. Clin. Psychol. 2016, 35, 822–830. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck depression inventory-II. Psychological Assessment 1996. J. Personal. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Steer, R.A.; Beck, A.T. Beck Anxiety Inventory. J. Consult. Clin. Psychol. 1988, 56, 893. [Google Scholar]
- Gorman, J.M. Comorbid depression and anxiety spectrum disorders. Depress. Anxiety 1996, 4, 160–168. [Google Scholar] [CrossRef]
- Sohn, S.I.; Kim, D.H.; Lee, M.Y.; Cho, Y.W. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012, 16, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.W.; Song, M.L.; Morin, C.M. Validation of a Korean version of the insomnia severity index. J. Clin. Neurol. 2014, 10, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Copay, A.G.; Subach, B.R.; Glassman, S.D.; Polly, D.W., Jr.; Schuler, T.C. Understanding the minimum clinically important difference: A review of concepts and methods. Spine J. 2007, 7, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Lichstein, K.L.; Morin, C.M. Recommendations for a standard research assessment of insomnia. Sleep 2006, 29, 1155–1173. [Google Scholar] [CrossRef]
- Troxel, W.M.; Conrad, T.S.; Germain, A.; Buysse, D.J. Predictors of treatment response to brief behavioral treatment of insomnia (BBTI) in older adults. J. Clin. Sleep Med. 2013, 9, 1281–1289. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Morin, C.M.; Schaefer, K.; Wallenstein, G.V. Interpreting score differences in the Insomnia Severity Index: Using health-related outcomes to define the minimally important difference. Curr. Med. Res. Opin. 2009, 25, 2487–2494. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, K.E.; Lee, S.A.; Jang, H.M.; Kim, D.H. Interplay between Human Gut Bacteria Escherichia coli and Lactobacillus mucosae in the Occurrence of Neuropsychiatric Disorders in Mice. Front. Immunol. 2020, 11, 273. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, C.; McCartney, D.; Desbrow, B.; Khalesi, S. Effects of probiotics and paraprobiotics on subjective and objective sleep metrics: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2020, 74, 1536–1549. [Google Scholar] [CrossRef] [PubMed]
- Talaei, A.; Hassanpour Moghadam, M.; Sajadi Tabassi, S.A.; Mohajeri, S.A. Crocin, the main active saffron constituent, as an adjunctive treatment in major depressive disorder: A randomized, double-blind, placebo-controlled, pilot clinical trial. J. Affect. Disord. 2015, 174, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Omenetti, S.; Pizarro, T.T. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front Immunol. 2015, 6, 639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godos, J.; Currenti, W.; Angelino, D.; Mena, P.; Castellano, S.; Caraci, F.; Galvano, F.; Del Rio, D.; Ferri, R.; Grosso, G. Diet and Mental Health: Review of the Recent Updates on Molecular Mechanisms. Antioxidants 2020, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef] [Green Version]
- Anukam, K.C.; Hayes, K.; Summers, K.; Reid, G. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 may help downregulate TNF-Alpha, IL-6, IL-8, IL-10 and IL-12 (p70) in the neurogenic bladder of spinal cord injured patient with urinary tract infections: A two-case study. Adv. Urol. 2009, 2009, 680363. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Park, S.; Paik, J.W.; Chae, S.W.; Kim, D.H.; Jeong, D.G.; Ha, E.; Kim, M.; Hong, G.; Park, S.H.; et al. Efficacy and Safety of Lactobacillus Plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghighat, N.; Rajabi, S.; Mohammadshahi, M. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: A randomized, double-blinded, clinical trial. Nutr. Neurosci. 2019, 24, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Tomasik, J.; Yolken, R.H.; Bahn, S.; Dickerson, F.B. Immunomodulatory Effects of Probiotic Supplementation in Schizophrenia Patients: A Randomized, Placebo-Controlled Trial. Biomark. Insights 2015, 10, 47–54. [Google Scholar] [CrossRef]
- Devasahayam, A.J.J.; Kelly, L.P.P.; Williams, J.B.B.; Moore, C.S.; Ploughman, M. Fitness Shifts the Balance of BDNF and IL-6 from Inflammation to Repair among People with Progressive Multiple Sclerosis. Biomolecules 2021, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.Y.; Zhang, X.; Yu, Z.H.; Zhang, Z.; Deng, M.; Zhao, J.H.; Ruan, B. Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res. 2018, 104, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Benatti, C.; Blom, J.M.; Rigillo, G.; Alboni, S.; Zizzi, F.; Torta, R.; Brunello, N.; Tascedda, F. Disease-Induced Neuroinflammation and Depression. CNS Neurol. Disord. Drug Targets 2016, 15, 414–433. [Google Scholar] [CrossRef]
- Peirce, J.M.; Alviña, K. The role of inflammation and the gut microbiome in depression and anxiety. J. Neurosci. Res. 2019, 97, 1223–1241. [Google Scholar] [CrossRef] [Green Version]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varghese, F.P.; Brown, E.S. The Hypothalamic-Pituitary-Adrenal Axis in Major Depressive Disorder: A Brief Primer for Primary Care Physicians. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 151–155. [Google Scholar] [CrossRef]
- Tafet, G.E.; Nemeroff, C.B. Pharmacological Treatment of Anxiety Disorders: The Role of the HPA Axis. Front Psychiatry 2020, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Rodenbeck, A.; Hajak, G. Neuroendocrine dysregulation in primary insomnia. Rev. Neurol. 2001, 157, S57–S61. [Google Scholar] [PubMed]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Characteristics | Experimental Group (n = 63) | Control Group (n = 59) | Total (n = 122) |
|---|---|---|---|
| Sex, male | 18 (28.6) | 21 (35.6) | 39 (31.97) |
| Age, years | 38.86 ± 10.89 | 37.63 ± 11.04 | 38.26 ± 10.94 |
| BMI, kg/m2 | 23.37 ± 3.31 | 23.44 ± 3.28 | 23.39 ± 3.28 |
| Medical illness *, presence | 6 (9.5) | 5 (8.5) | 11 (9.0) |
| Medication use †, presence | 7 (11.1) | 4 (6.8) | 11 (9.0) |
| Occupation | |||
| Office worker | 20 (31.7) | 13 (22.0) | 33 (27.1) |
| Professionals | 15 (23.8) | 13 (22.0) | 28 (22.9) |
| Students | 3 (4.8) | 4 (6.8) | 7 (5.7) |
| Others | 6 (9.5) | 8 (13.6) | 14 (11.5) |
| No job | 19 (30.2) | 21 (35.6) | 40 (32.8) |
| Physical activity ‡, frequency | |||
| <1/week | 28 (44.4) | 25 (42.4) | 53 (43.4) |
| 1~2/week | 23 (36.5) | 21 (35.6) | 44 (36.1) |
| 3~4/week | 9 (14.3) | 11 (18.6) | 20 (16.4) |
| >4/week | 3 (4.8) | 2 (3.4) | 5 (4.1) |
| Smoking history § | |||
| Non-smoker | 59 (93.7) | 51 (86.4) | 110 (90.2) |
| Ex-smoker (no smoking for > 6 months) | 1 (1.6) | 2 (3.4) | 3 (2.5) |
| Smoker | 3 (4.8) | 6 (10.2) | 9 (7.4) |
| Alcohol ‖ | |||
| No | 41 (65.1) | 33 (55.9) | 74 (60.1) |
| <6 units/week | 16 (25.4) | 19 (32.2) | 35 (28.7) |
| 6~12 units/week | 6 (9.5) | 5 (8.5) | 11 (9.0) |
| >12 units/week | 0 (0.00) | 2 (3.4) | 2 (1.6) |
| Questionnaires, score | |||
| SRI | 69.57 ± 14.09 | 66.22 ± 12.65 | 67.95 ± 13.46 |
| BDI-II | 26.05 ± 7.90 | 23.97 ± 5.35 | 25.04 ± 6.83 |
| BAI | 16.35 ± 9.64 | 14.97 ± 7.09 | 15.68 ± 8.49 |
| PSQI | 8.16 ± 3.04 | 7.22 ± 2.29 | 7.72 ± 2.77 |
| ISI | 11.46 ± 4.54 | 10.03 ± 4.84 | 10.73 ± 4.76 |
| Characteristics | Experimental Group (n = 63) | Control Group (n = 59) | p † | ||||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD | ES | n | Mean ± SD | ES | ||
| SRI, score | |||||||
| Baseline | 63 | 69.57 ± 14.09 | 59 | 66.22 ± 12.65 | |||
| Week 4 | 61 | 49.13 ± 19.34 | 58 | 47.57 ± 18.91 | |||
| Δ | −20.27 ± 18.63 | 1.21 | −18.90 ± 21.24 | 1.16 | 0.750 | ||
| p-value ‡ | <0.001 | <0.001 | |||||
| Week 8 | 63 | 41.68 ± 20.17 | 59 | 41.76 ± 21.27 | |||
| Δ | −27.89 ± 24.46 | 1.60 | −24.46 ± 25.95 | 1.40 | 0.454 | ||
| p-value ‡ | <0.001 | <0.001 | |||||
| BDI-II, score | |||||||
| Baseline | 63 | 26.05 ± 7.90 | 59 | 23.97 ± 5.35 | |||
| Week 4 | 62 | 19.89 ± 6.93 | 57 | 20.47 ± 6.68 | |||
| Δ | −6.18 ± 7.34 | 0.83 | −3.33 ± 7.03 | 0.58 | 0.033 | ||
| p-value ‡ | <0.001 | 0.007 | |||||
| Week 8 | 63 | 18.03 ± 6.90 | 59 | 18.58 ± 6.46 | |||
| Δ | −8.02 ± 7.17 | 1.08 | −5.39 ± 6.49 | 0.91 | 0.036 | ||
| p-value ‡ | <0.001 | <0.001 | |||||
| BAI, score | |||||||
| Baseline | 63 | 16.35 ± 9.64 | 59 | 14.97 ± 7.09 | |||
| Week 4 | 62 | 11.60 ± 7.16 | 57 | 13.47 ± 7.93 | |||
| Δ | −4.73 ± 7.32 | 0.56 | −1.37 ± 7.27 | 0.20 | 0.014 | ||
| p-value ‡ | <0.001 | 0.161 | |||||
| Week 8 | 63 | 11.05 ± 7.48 | 59 | 12.03 ± 7.90 | |||
| Δ | −5.30 ± 8.34 | 0.61 | −2.93 ± 8.08 | 0.39 | 0.114 | ||
| p-value ‡ | <0.001 | 0.007 | |||||
| Characteristics | Experimental Group (n = 63) | Control Group (n = 59) | p † | ||||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD | ES | n | Mean ± SD | ES | ||
| PSQI, score | |||||||
| Baseline | 63 | 8.16 ± 3.04 | 59 | 7.22 ± 2.29 | |||
| Week 4 | 62 | 7.61 ± 2.64 | 58 | 6.90 ± 2.76 | |||
| Δ | −0.63 ± 2.78 | 0.19 | −0.33 ± 2.54 | 0.13 | 0.537 | ||
| p-value ‡ | 0.080 | 0.331 | |||||
| Week 8 | 63 | 6.83 ± 2.79 | 59 | 6.80 ± 2.36 | |||
| Δ | −1.33 ± 3.03 | 0.46 | −0.42 ± 2.36 | 0.18 | 0.068 | ||
| p-value ‡ | 0.001 | 0.174 | |||||
| ISI, score | |||||||
| Baseline | 63 | 11.46 ± 4.54 | 59 | 10.03 ± 4.84 | |||
| Week 4 | 61 | 9.80 ± 4.63 | 58 | 9.64 ± 4.88 | |||
| Δ | −1.77 ± 3.95 | 0.36 | −0.43 ± 5.31 | 0.08 | 0.123 | ||
| p-value ‡ | 0.001 | 0.539 | |||||
| Week 8 | 63 | 8.19 ± 4.17 | 59 | 8.90 ± 4.32 | |||
| Δ | −3.27 ± 3.89 | 0.75 | −1.14 ± 4.55 | 0.25 | 0.006 | ||
| p-value ‡ | <0.001 | 0.060 | |||||
| PSQI Subscales | Experimental Group (n = 63) | Control Group (n = 59) | p † | p ‡ |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| PSQI total | ||||
| Baseline | 8.16 ± 3.04 | 7.22 ± 2.29 | ||
| Week 8 | 6.83 ± 2.79 | 6.80 ± 2.36 | ||
| Δ | −1.33 ± 3.03 | −0.42 ± 2.36 | 0.068 | 0.020 |
| p-value § | 0.001 | 0.174 | ||
| Sleep quality | ||||
| Baseline | 1.60 ± 0.58 | 1.54 ± 0.54 | ||
| Week 8 | 1.38 ± 0.61 | 1.36 ± 0.48 | ||
| Δ | −0.22 ± 0.66 | −0.19 ± 0.60 | 0.755 | 0.794 |
| p-value § | 0.010 | 0.021 | ||
| Sleep latency | ||||
| Baseline | 1.84 ± 0.90 | 1.66 ± 0.73 | ||
| Week 8 | 1.40 ± 0.79 | 1.54 ± 0.79 | ||
| Δ | −0.44 ± 0.76 | −0.12 ± 0.88 | 0.024 | 0.018 |
| p-value § | <0.001 | 0.266 | ||
| Sleep duration | ||||
| Baseline | 1.17 ± 1.07 | 0.85 ± 0.85 | ||
| Week 8 | 1.10 ± 0.93 | 0.81 ± 0.92 | ||
| Δ | −0.08 ± 1.13 | −0.03 ± 0.95 | 0.810 | 0.307 |
| p-value § | 0.578 | 0.784 | ||
| Sleep efficiency | ||||
| Baseline | 0.43 ± 0.87 | 0.31 ± 0.62 | ||
| Week 8 | 0.32 ± 0.67 | 0.42 ± 0.75 | ||
| Δ | −0.11 ± 0.92 | 0.12 ± 0.85 | 0.270 | 0.108 |
| p-value § | 0.340 | 0.290 | ||
| Sleep disturbances | ||||
| Baseline | 1.35 ± 0.54 | 1.25 ± 0.51 | ||
| Week 8 | 1.21 ± 0.48 | 1.12 ± 0.46 | ||
| Δ | −0.14 ± 0.53 | −0.14 ± 0.54 | 0.941 | 0.995 |
| p-value § | 0.038 | 0.059 | ||
| Use of sleep pills | ||||
| Baseline | 0.02 ± 0.13 | 0.02 ± 0.13 | ||
| Week 8 | 0.06 ± 0.30 | 0.08 ± 0.34 | ||
| Δ | 0.05 ± 0.33 | 0.07 ± 0.31 | 0.732 | 0.570 |
| p-value § | 0.260 | 0.103 | ||
| Daytime dysfunction | ||||
| Baseline | 1.75 ± 0.76 | 1.59 ± 0.65 | ||
| Week 8 | 1.37 ± 0.79 | 1.46 ± 0.73 | ||
| Δ | −0.38 ± 0.99 | −0.14 ± 0.75 | 0.125 | 0.062 |
| p-value § | 0.003 | 0.172 |
| Biomarkers | Experimental Group (n = 63) | Control Group (n = 59) | p† | ||
|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | ||
| IL-6, pg/mL | |||||
| Baseline | 63 | 1.47 ± 1.08 | 59 | 1.29 ± 0.75 | |
| Week 8 | 59 | 1.25 ± 0.62 | 59 | 1.49 ± 1.32 | |
| Δ | −0.23 ± 1.06 | 0.20 ± 1.20 | 0.041 | ||
| p-value ‡ | 0.105 | 0.200 | |||
| TNF-alpha, pg/mL | |||||
| Baseline | 63 | 0.72 ± 0.32 | 59 | 0.79 ± 0.34 | |
| Week 8 | 59 | 0.74 ± 0.20 | 59 | 0.79 ± 0.33 | |
| Δ | 0.02 ± 0.32 | 0.00 ± 0.32 | 0.704 | ||
| p-value ‡ | 0.602 | 0.990 | |||
| BDNF, log pg/mL | |||||
| Baseline | 63 | 10.02 ± 0.32 | 59 | 10.03 ± 0.31 | |
| Week 8 | 59 | 10.10 ± 0.23 | 59 | 10.11 ± 0.27 | |
| Δ | 0.09 ± 0.25 | 0.08 ± 0.24 | 0.734 | ||
| p-value ‡ | 0.007 | 0.017 | |||
| Cortisol, µg/dL | |||||
| Baseline | 63 | 7.57 ± 4.13 | 59 | 8.28 ± 3.72 | |
| Week 8 | 59 | 7.38 ± 2.46 | 59 | 7.69 ± 3.08 | |
| Δ | −0.18 ± 4.50 | −0.59 ± 3.89 | 0.597 | ||
| p-value ‡ | 0.757 | 0.247 | |||
| ACTH, pg/mL | |||||
| Baseline | 63 | 18.33 ± 14.23 | 59 | 19.29 ± 13.68 | |
| Week 8 | 59 | 22.29 ± 13.61 | 59 | 24.23 ± 28.32 | |
| Δ | 3.66 ± 15.95 | 4.94 ± 23.96 | 0.734 | ||
| p-value ‡ | 0.083 | 0.119 | |||
| IL-6/log (BDNF) | |||||
| Baseline | 63 | 0.15 ± 0.11 | 59 | 0.13 ± 0.08 | |
| Week 8 | 59 | 0.12 ± 0.06 | 59 | 0.15 ± 0.13 | |
| Δ | −0.02 ± 0.11 | 0.02 ± 0.12 | 0.041 | ||
| p-value ‡ | 0.089 | 0.225 | |||
| TNF-alpha/log (BDNF) | |||||
| Baseline | 63 | 0.07 ± 0.03 | 59 | 0.08 ± 0.03 | |
| Week 8 | 59 | 0.07 ± 0.02 | 59 | 0.08 ± 0.03 | |
| Δ | 0.00 ± 0.03 | −0.00 ± 0.03 | 0.722 | ||
| p-value ‡ | 0.707 | 0.902 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.J.; Hong, J.K.; Kim, J.-K.; Kim, D.-H.; Jang, S.W.; Han, S.-W.; Yoon, I.-Y. Effects of Probiotic NVP-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2660. https://doi.org/10.3390/nu13082660
Lee HJ, Hong JK, Kim J-K, Kim D-H, Jang SW, Han S-W, Yoon I-Y. Effects of Probiotic NVP-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2021; 13(8):2660. https://doi.org/10.3390/nu13082660
Chicago/Turabian StyleLee, Hyuk Joo, Jung Kyung Hong, Jeon-Kyung Kim, Dong-Hyun Kim, Seok Won Jang, Seung-Won Han, and In-Young Yoon. 2021. "Effects of Probiotic NVP-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 13, no. 8: 2660. https://doi.org/10.3390/nu13082660