Fructo-Oligosaccharides and Pectins Enhance Beneficial Effects of Raspberry Polyphenols in Rats with Nonalcoholic Fatty Liver
Abstract
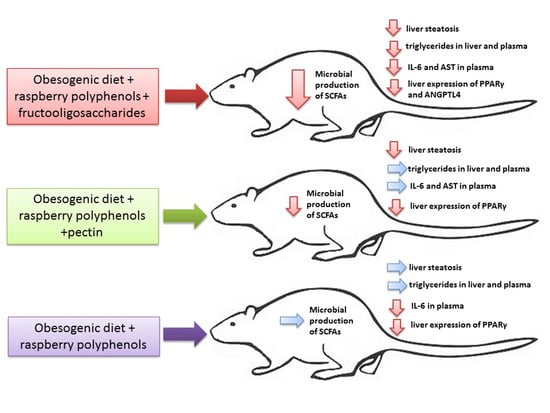
:1. Introduction
2. Materials and Methods
2.1. Raspberry Polyphenolic Extract (RE) Production
2.2. Polyphenolic Analysis
2.3. Animals and Experimental Design
2.4. Collection of Biological Material and Analyticla Procedures
2.5. Caecal Microbial SCFAs
2.6. Plasma Lipid Profile and Inflammatory Markers
2.7. Liver Histopathology
2.8. Hepatic Gene Expression
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Astrup, A.; Dyerberg, J.; Elwood, P.; Hermansen, K.; Hu, F.B.; Jakobsen, M.U.; Kok, F.J.; Krauss, R.M.; Lecerf, J.M.; LeGrand, P.; et al. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: Where does the evidence stand in 2010? Am. J. Clin. Nutr. 2011, 93, 684–688. [Google Scholar] [CrossRef] [Green Version]
- Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig. Liver Dis. 2017, 49, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milic, N.; Luzza, F.; Boccuto, L.; De Lorenzo, A. Polyphenols treatment in patients with nonalcoholic fatty liver disease. J. Transl. Int. Med. 2017, 5, 144–147. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Montes de Oca, A.; Julián, M.T.; Ramos, A.; Puig-Domingo, M.; Alonso, N. Microbiota, fiber, and NAFLD: Is there any connection? Nutrients 2020, 12, 3100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sandhu, A.; Edirisinghe, I.; Burton-Freeman, B. An exploratory study of red raspberry (Rubus idaeus L.) (poly)phenols/metabolites in human biological samples. Food Funct. 2018, 21, 806–818. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Weber, C.; Lee, C.; Brown, J.; Liu, R. Antioxidant and antiproliferative activities of raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef] [PubMed]
- Bobinaitė, R.; Viškelis, P.; Šarkinas, A.; Venskutonis, P.R. Phytochemical composition, antioxidant and antimicrobial properties of raspberry fruit, pulp, and marc extracts. CyTA J. Food 2013, 11, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Fotschki, B.; Juśkiewicz, J.; Sójka, M.; Jurgoński, A.; Zduńczyk, Z. Ellagitannins and flavan-3-ols from raspberry pomace modulate caecal fermentation processes and plasma lipid parameters in rats. Molecules 2015, 21, 22848–22862. [Google Scholar] [CrossRef] [Green Version]
- Fotschki, B.; Laparra, J.M.; Sójka, M. Raspberry polyphenolic extract regulates obesogenic signals in hepatocytes. Molecules 2018, 23, 2103. [Google Scholar] [CrossRef] [Green Version]
- Barberan, F.A.; Garcia-Conesa, M.T.; Larrosa, M.; Cerdá, B.; Gonzalez-Barrio, R.; Bermudez-Soto, M.J.; González-Sarrías, A.; Espín, J.C. Bioavailability, metabolism, and bioactivity of food ellagic acid and related polyphenols. Recent Adv. Polyphenol. Res. 2008, 1, 263–277. [Google Scholar]
- Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z. Comparative effects of different dietary levels of cellulose and fructooligosaccharides on fermentative processes in the caecum of rats. J. Anim. Feed Sci. 2008, 17, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Li, S.; Luo, J.; Wu, X.; Liu, L. Effects of dietary fibers and their mixtures on short chain fatty acids and microbiota in mice guts. Food Funct. 2013, 4, 932–938. [Google Scholar] [CrossRef]
- Fotschki, B.; Milala, J.; Jurgoński, A.; Karlińska, E.; Zduńczyk, Z.; Juśkiewicz, J. Strawberry ellagitannins thwarted the positive effects of dietary fructooligosaccharides in rat cecum. J. Agric. Food Chem. 2014, 62, 5871–5880. [Google Scholar] [CrossRef]
- Nobili, V.; Alkhouri, N.; Bartuli, A.; Manco, M.; Lopez, R.; Alisi, A.; Feldstein, A.E. Severity of liver injury and atherogenic lipid profile in children with nonalcoholic fatty liver disease. Pediatr. Res. 2010, 67, 665–670. [Google Scholar] [CrossRef] [Green Version]
- Juśkiewicz, J.; Milala, J.; Jurgoński, A.; Król, B.; Zduńczyk, Z. Consumption of polyphenol concentrate with dietary fructo-oligosaccharides enhances cecal metabolism of quercetin glycosides in rats. Nutrition 2010, 27, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Klewicka, E.; Sójka, M.; Klewicki, R.; Kołodziejczyk, K.; Lipińska, L.; Nowak, A. Ellagitannins from raspberry (Rubus idaeus L.) fruit as natural inhibitors of Geotrichum candidum. Molecules 2016, 21, 908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sójka, M.; Klimczak, E.; Macierzyński, J.; Kołodziejczyk, K. Nutrient and polyphenolic composition of industrial strawberry press cake. Eur. Food Res. Technol. 2013, 237, 995–1007. [Google Scholar] [CrossRef] [Green Version]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838–841. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Jurgoński, A.; Juśkiewicz, J.; Fotschki, B.; Kołodziejczyk, K.; Milala, J.; Kosmala, M.; Grzelak-Błaszczyk, K.; Markiewicz, L. Metabolism of strawberry mono- and dimeric ellagitannins in rats fed a diet containing fructo-oligosaccharides. Eur. J. Nutr. 2017, 56, 853–864. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.J.; Fan, J.G.; Ding, X.D.; Qiao, L.; Wang, G.L. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig. Dis. Sci. 2010, 55, 931–940. [Google Scholar] [CrossRef] [Green Version]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Opyd, P.M.; Jurgoński, A.; Juśkiewicz, J.; Fotschki, B.; Koza, J. Comparative effects of native and defatted flaxseeds on intestinal enzyme activity and lipid metabolism in rats fed a high-fat diet containing cholic acid. Nutrients 2018, 10, 1181. [Google Scholar] [CrossRef] [Green Version]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Red raspberries and their bioactive polyphenols: Cardiometabolic and neuronal health links. Adv. Nutr. 2016, 1, 44–65. [Google Scholar] [CrossRef] [Green Version]
- Kshatriya, D.; Li, X.; Giunta, G.M.; Yuan, B.; Zhao, D.; Simon, J.E.; Wu, Q.; Bello, N.T. Phenolic-enriched raspberry fruit extract (Rubus idaeus) resulted in lower weight gain, increased ambulatory activity, and elevated hepatic lipoprotein lipase and heme oxygenase-1 expression in male mice fed a high-fat diet. Nutr. Res. 2019, 68, 19–33. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [Green Version]
- Puljula, E.; Walton, G.; Woodward, M.J.; Karonen, M. Antimicrobial activities of ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules 2020, 25, 3714. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Perry, R.; Peng, L.; Barry, N.; Cline, G.; Zhang, D.; Cardone, R.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Tirosh, A.; Calay, E.S.; Tuncman, G.; Claiborn, K.C.; Inouye, K.E.; Eguchi, K.; Alcala, M.; Rathaus, M.; Hollander, K.S.; Ron, I.; et al. The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. Sci. Transl. Med. 2019, 11, eaav0120. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short chain fatty acids and fecal microbiota abundance in humans with obesity: A systematic review and meta-Analysis. Nutrients 2019, 11, 2512. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Juśkiewicz, J.; Zduńczyk, Z.; Żary-Sikorska, E.; Król, B.; Milala, J.; Jurgoński, A. Effect of the dietary polyphenolic fraction of chicory root, peel, seed and leaf extracts on caecal fermentation and blood parameters in rats fed diets containing prebiotic fructans. Br. J. Nutr. 2011, 105, 710–720. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Natsume, M.; Yasuda, A.; Ishizaka, M.; Kawahata, K.; Koga, J. Fructooligosaccharides suppress high-fat diet-induced fat accumulation in C57BL/6J mice. Biofactors 2017, 43, 145–151. [Google Scholar] [CrossRef]
- Bray, J.K.; Chiu, G.S.; McNeil, L.K.; Moon, M.L.; Wall, R.; Towers, A.E.; Freund, G.G. Switching from a high-fat cellulose diet to a high-fat pectin diet reverses certain obesity-related morbidities. Nutr. Metab. 2018, 15, 55. [Google Scholar] [CrossRef]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef]
- Than, N.N.; Newsome, P.N. A concise review of non-alcoholic fatty liver disease. Atherosclerosis 2015, 239, 192–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, Y.; Nagaike, H. Increased angiopoietin like protein 4 (Angptl4) is associated with higher concentration of LDL-triglycerides in type 2 Diabetes. Atherosclerosis 2019, 287, 80–81. [Google Scholar] [CrossRef]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as metabolic regulators in the liver: Lessons from liver-specific PPAR-null mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, 47–64. [Google Scholar] [CrossRef] [Green Version]
- Kanga, I.; Espín, J.C.; Carra, T.P.; Tomás-Barberán, F.A.; Chunga, S. Raspberry seed flour attenuates high-sucrose diet-mediated hepatic stress and adipose tissue inflammation. J. Biol. Chem. 2016, 32, 64–72. [Google Scholar] [CrossRef] [Green Version]



| Compound | mg/100 g |
|---|---|
| Ellagitannins (ET) n = 3 | |
| Lambertianin C | 18,314.0 ± 1172.6 |
| Lambertianin C minus ellagic acid moiety 1 a | 764.1 ± 28.8 |
| Lambertianin C minus ellagic acid moiety 2 a | 196.4 ± 10.9 |
| Lambertianin C minus ellagic acid moiety 3 a | 426.6 ± 6.7 |
| Sanguiin H-6 | 16,975.8 ± 350.4 |
| Sanguiin H-6 minus gallic acid moiety b | 221.6 ± 9.7 |
| Sanguiin H-6 plus gallic acid moiety b | 356.3 ± 22.4 |
| Sanguiin H-10 isomer 1 b | 466.4 ± 14.0 |
| Sanguiin H-10 isomer 2 b | 533.0 ± 20.7 |
| Sanguiin H-10 isomer 3 b | 301.2 ± 10.7 |
| Ellagic acid pentose conjugate c | 171.7 ± 11.4 |
| Ellagic acid | 196.7 ± 18.1 |
| Total ET | 38,088.9 ± 1503.6 |
| Total EAC | 368.3 ± 29.4 |
| Total ET + EAC | 38,923.6 ± 1547.0 |
| Flavanols (FLAVA) n = 3 | |
| Total FLAVA | 8371.7 ± 486.5 |
| (+)-Catechin | 208.0 ± 5.4 |
| (−)-Epicatechin | 343.5 ± 5.5 |
| Proanthocyanidins | 7820.2 ± 475.7 |
| Extension units (%) | |
| (+)-Catechin | 28.5 ± 0.1 |
| (−)-Epicatechin | 3.0 ± 0.0 |
| Terminal units (%) | |
| (+)-Catechin | 13.5 ± 0.0 |
| (−)-Epicatechin | 55.0 ± 0.1 |
| mDP (°) | 1.5 ± 0.0 |
| Anthocyanins (ACY) n = 3 | |
| Cyanidin-3-O-spohoroside d | 314.6 ± 11.5 |
| Cyanidin-3-O-glucosyl-rutinoside d | 27.0 ± 0.7 |
| Cyanidin-3-O-glucoside | 152.0 ± 0.9 |
| Cyanidin-3-O-rutinoside d | 11.5 ± 0.1 |
| Pelargonidin-3-O-glucoside d | 4.5 ± 0.3 |
| Total ACY | 509.6 ± 10.9 |
| Total polyphenols (TPH) | 47,804.8 ± 1060.5 |
| Parameters | Groups | ANOVA p Value | |||
|---|---|---|---|---|---|
| H | HP | HPF | HPP | ||
| Diet intake (g/day) | 25.32 ± 2.11 | 24.82 ± 1.56 | 23.92 ± 2.88 | 25.02 ± 1.31 | NS |
| Final body weight (g) | 466.64 ± 11.91 | 472.74 ± 14.22 | 465.43 ± 9.21 | 457.94 ± 15.37 | NS |
| Final body fat (g) | 137.62 ± 4.42 | 142.69 ± 9.98 | 130.05 ± 6.46 | 131.48 ± 5.44 | NS |
| Final body lean (g) | 253.43 ± 6.82 | 254.48 ± 5.53 | 258.05 ± 5.19 | 254.96 ± 6.65 | NS |
| Epididymal fat (g) | 20.54 ± 1.37 | 21.62 ± 2.17 | 18.64 ± 1.45 | 19.31 ± 1.37 | NS |
| Small intestine: | |||||
| Tissue mass (g) | 6.14 ± 0.35 | 6.62 ± 0.19 | 6.99 ± 0.27 | 6.72 ± 0.16 | NS |
| pH | 7.01 ± 0.08 | 6.83 ± 0.08 | 6.81 ± 0.078 | 6.71 ± 0.10 | NS |
| Caecum: | |||||
| Tissue mass (g) | 0.56 ± 0.03 b | 0.62 ± 0.02 ab | 0.69 ± 0.03 a | 0.66 ± 0.02 a | <0.01 |
| pH | 7.08 ± 0.08 a | 6.91 ± 0.09 ab | 7.08 ± 0.08 a | 6.70 ± 0.13 b | <0.05 |
| SCFA (µmol/g digesta) | |||||
| Acetic acid | 26.38 ± 1.85 a | 27.13 ± 4.12 a | 17.28 ± 1.71 b | 21.95 ± 1.79 ab | <0.05 |
| Propionic acid | 7.84 ± 0.34 | 8.03 ± 0.41 | 5.83 ± 0.60 | 6.95 ± 0.87 | 0.054 |
| Iso-butyric acid | 0.63 ± 0.04 a | 0.60 ± 0.07 ab | 0.44 ± 0.04 b | 0.40 ± 0.06 c | <0.05 |
| Butyric acid | 1.86 ± 0.19 a | 1.80 ± 0.24 a | 0.93 ± 0.17 b | 1.52 ± 0.12 a | <0.01 |
| Iso-valeric acid | 0.78 ± 0.05 a | 0.78 ± 0.06 a | 0.57 ± 0.05 b | 0.55 ± 0.06 b | <0.01 |
| Valeric acid | 0.62 ± 0.03 a | 0.58 ± 0.05 ab | 0.44 ± 0.05 b | 0.26 ± 0.05 c | <0.001 |
| SCFA profile (%) | |||||
| Acetic acid | 68.90 ± 0.95 | 67.92 ± 2.72 | 67.64 ± 1.33 | 69.63 ± 1.87 | NS |
| Propionic acid | 20.82 ± 0.85 | 21.79 ± 1.71 | 22.84 ± 1.19 | 21.81 ± 1.69 | NS |
| Butyric acid | 4.85 ± 0.37 | 4.66 ± 0.40 | 3.60 ± 0.54 | 4.90 ± 0.32 | NS |
| Total PSCFAs | 2.03 ± 0.10 a | 1.98 ± 0.16 a | 1.48 ± 0.15 ab | 1.22 ± 0.18 b | <0.01 |
| Total SCFAs | 38.12 ± 2.24 a | 38.94 ± 4.62 a | 25.53 ± 2.38 b | 31.64 ± 2.65 ab | <0.05 |
| Parameters | Groups | ANOVA p Value | |||
|---|---|---|---|---|---|
| H | HP | HPF | HPP | ||
| TG (mmol/L) | 1.05 ± 0.10 a | 0.89 ± 0.11 a | 0.64 ± 0.05 b | 0.70 ± 0.06 a | <0.05 |
| TC (mmol/L) | 2.99 ± 0.24 | 2.69 ± 0.20 | 2.97 ± 0.21 | 2.40 ± 0.30 | NS |
| HDL (mmol/L) | 0.42 ± 0.02 | 0.44 ± 0.02 | 0.39 ± 0.03 | 0.36 ± 0.03 | NS |
| non-HDL (mmol/L) | 2.58 ± 0.25 | 2.25 ± 0.20 | 2.59 ± 0.22 | 2.04 ± 0.31 | NS |
| Parameters | Groups | ANOVA p Value | |||
|---|---|---|---|---|---|
| H | HP | HPF | HPP | ||
| Liver (g) | 15.93 ± 0.50 b | 17.10 ± 0.75 ab | 18.71 ± 0.51 a | 16.21 ± 0.71 b | <0.05 |
| Fat content (%) | 34.45 ± 1.47 a | 31.13 ± 1.87 a | 27.25 ± 1.24 b | 27.57 ± 1.27 b | <0.01 |
| Cholesterol (mg/g liver) | 10.38 ± 0.41 a | 8.73 ± 1.02 a | 7.63 ± 0.76 b | 8.54 ± 0.99 a | <0.05 |
| TG (mg/g liver) | 19.42 ± 0.85 a | 16.70 ± 1.80 ab | 14.81 ± 0.48 b | 17.75 ± 1.03 ab | <0.05 |
| MDA (ng/g liver) | 781.1 ± 25.4 a | 680.6 ± 21.0 b | 642.5 ± 16.9 b | 752.1 ± 35.9 a | <0.01 |
| Plasma indicators (U/L) | |||||
| AST | 204.1 ± 12.7 a | 141.9 ± 18.9 bc | 134.6 ± 8.9 c | 167.6 ± 25.2 ab | <0.05 |
| ALT | 122.2 ± 45.2 | 121.7 ± 34.1 | 108.7 ± 27.0 | 97.1 ± 29.5 | NS |
| ALP | 92.8 ± 8.8 | 85.6 ± 10.5 | 104.2 ± 3.9 | 89.7 ± 5.2 | NS |
| Parameters | Stage 1 | ANOVA p Value | ||
|---|---|---|---|---|
| Group | Low | High | ||
| Steatosis, n (%) | H a | 0 (0) | 8 (100) | <0.01 |
| HP a | 2 (25) | 6 (75) | ||
| HPF b | 7 (87.5) | 1 (12.5) | ||
| HPP b | 6 (75) | 2 (25) | ||
| Ballooning, n (%) | H a | 0 (0) | 8 (100) | <0.01 |
| HP b | 4 (50) | 4 (50) | ||
| HPF c | 8 (100) | 0 (0) | ||
| HPP c | 8 (100) | 0 (0) | ||
| Lobular inflammation, n (%) | H a | 0 (0) | 8 (100) | <0.05 |
| HP b | 3 (37.5) | 5 (62.5) | ||
| HPF c | 7 (87.5) | 1 (12.5) | ||
| HPP ab | 2 (25) | 6 (75) | ||
| Portal inflammation, n (%) | H a | 1 (12.5) | 7 (87.5) | <0.05 |
| HP b | 3 (37.5) | 5 (62.5) | ||
| HPF c | 6 (75) | 2 (25) | ||
| HPP bc | 4 (50) | 4 (50) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Sójka, M. Fructo-Oligosaccharides and Pectins Enhance Beneficial Effects of Raspberry Polyphenols in Rats with Nonalcoholic Fatty Liver. Nutrients 2021, 13, 833. https://doi.org/10.3390/nu13030833
Fotschki B, Juśkiewicz J, Jurgoński A, Sójka M. Fructo-Oligosaccharides and Pectins Enhance Beneficial Effects of Raspberry Polyphenols in Rats with Nonalcoholic Fatty Liver. Nutrients. 2021; 13(3):833. https://doi.org/10.3390/nu13030833
Chicago/Turabian StyleFotschki, Bartosz, Jerzy Juśkiewicz, Adam Jurgoński, and Michał Sójka. 2021. "Fructo-Oligosaccharides and Pectins Enhance Beneficial Effects of Raspberry Polyphenols in Rats with Nonalcoholic Fatty Liver" Nutrients 13, no. 3: 833. https://doi.org/10.3390/nu13030833