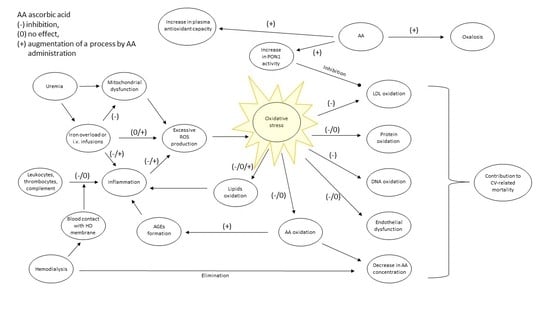
Two Faces of Vitamin C in Hemodialysis Patients: Relation to Oxidative Stress and Inflammation
Abstract
:1. Introduction
2. Sources of Inflammation and Oxidative Stress in Hemodialysis Patients
3. Vitamin C potential Against Inflammation and Oxidative Stress
4. Concentration of Ascorbic Acid in Plasma and Serum of Hemodialysis Patients
| Dialysis Modality (Study’s Country) | Study Description | Main Results | References |
|---|---|---|---|
| Hemodialysis (Greece) | Cross-sectional, 93 HD patients (aged 19–71 years, HD dose 12 h/week, HD vintage 10–137 months), 52 healthy controls, AA determination with the enzymatic method in pre-HD serum. | HD patients presented 4-fold smaller mean AA concentration than healthy subjects, 12.0 ± 8.1 vs. 50.0 ± 22.1 μmol/L respectively. | Papastephanidis et al.(1987) [35] |
| Hemodiafiltration or hemodialysis (Austria) | Cross-sectional, 130 HD/HDF patients (median: age 60, 74% on HDF, HD/HDF vintage 20 months, Kt/V 1.39). AA determination with the HPLC method in pre-HD plasma. | Median AA concentration was 45.1 μmol/L, IQR: 24.3–76.1 μmol/L. | Deicher et al.(2004) [42] |
| Hemodialysis (NYC, USA) | Cross-sectional, 117 HD patients (mean: age 63, HD vintage 3.2 years, HD dose unknown). AA determination with the HPLC in pre-HD plasma m | Mean AA concentration was 58.9 ± 65.3 μmol/L. Values < 10 μmol/L, between 10 and 80 μmol/L and > 80 μmol/L observed in 15%, 66% and 19% percent of patients respectively. | Richter et al.(2008) [45] |
| Hemodialysis (China) | Cross-sectional, 117 HD patients (mean: age 59, HD vintage 49 months, Kt/V 1.6) AA determination with the HPLC in pre-HD plasma m. | AA concentration < 2 μg/mL **, between 2- 4 μg/mL and > 4 μg/mL was present in: 38%, 27% and 35% of patients respectively | Zhang et al. (2011) [46] |
| Conventional and extended hours hemodialysis (Australia) | Cross-sectional, 26 patients in EHD (mean age: 55, HD vintage 25.7 months, HD dose 28 h/week) 26 in CHD (mean: age 71, HD vintage 57 months, HD dose 13.5 h/week). AA determination with HPLC in pre-HD serum. | Median pre-HD AA concentration was 3.8-fold lower in the EHD than the CHD group (0.30 vs. 1.14 mg/dL *). | Coveney et al.(2011) [48] |
| Conventional and frequent hemodialysis (USA) | Participants of the FHN trial, NCT00264758. CHD group: 20 patients, mean age 50.6, 3 HD sessions per week, median duration 3.5 h, Kt/V = 1.46. FHD group: 24 patients, mean age 48.8, 6 HD sessions per week, median duration 2.4 h, Kt/V = 1.43. AA determination with the HPLC in pre-HD plasma m. | Mean AA concentration approximated 25 ± 22 μmol/L and did not differ between groups and time points. 25% of patients have AA concentration < 10 μmol/L. | Raimann et al. (2019) [47] |
5. Influence of Ascorbic Acid Supplementation on Its Concentration in Serum and Plasma of Hemodialysis Patients
| Objective (Study’s Country) | Study Description | Main Results | References |
|---|---|---|---|
| Evaluation of the effects of oral AA supplementation on AA concentration in plasma. (Turkey) | Prospective, open label, randomized, placebo-controlled trial, 34 HD patients (mean age 46, HD vintage unknown, dose 12 h/week) received orally 250 mg of AA/day for 90 days (n = 15) or placebo. The concentration of AA in pre-HD plasma was measured with fluorimetry. | AA concentration increased from 32 ± 13 to 46 ± 16 μmol/L, remaining unchanged after placebo. | Candan et al. (2002) [10] |
| Evaluation of the influence of intravenous AA supplementation on AA concentration in plasma. (Taiwan) | Prospective, open label, randomized, placebo-controlled trial, 51 HD patients (mean: age 59, HD vintage 46 months, HD dose 12–13.5 h/week) 26 in the placebo group, 300 mg of AA or saline was infused after each HD for 8 weeks, pre-HD AA in plasma m was measured with colorimetric method. | AA concentration doubled from initial 44 ± 19 μmol/L, remaining unchanged after placebo. | Tarng et al. (2004) [21] |
| Assessment of the effects of intravenous AA supplementation on AA concentration in plasma. (Italy) | Prospective observational study; 14 HD and 4 HDF patients with AA deficiency (mean: HD vintage 9.9 years, Kt/V ≥ 1.2) AA was infused once a week after HD in a dose of 250 mg for 3 months and 500 mg for the following year. AA determination with HPLC in pre-HD plasma m samples. | Mean concentration of AA raised from initial 1.6 ± 0.8 mg/L # to maximal 6.6 ± 2.8 mg/L. | Canavese et al. (2005) [39] |
| Evaluation of the effects of intravenous or enteral AA supplementation on AA concentration in plasma. (Australia) | Prospective, randomized, parallel observational study, 21 HD patients (mean: age 56, HD vintage > 6 months, sessions thrice a week, URR 76%) received 250 mg of AA orally or IV after HD sessions for 8 weeks. AA measurements with HPLC in pre-HD plasma. | Mean concentration of AA increased 2.8 fold from initial 2.8 ± 0.7 mg/L # in the enteral group and 3.4 fold from initial 1.8 ± 0.5 mg/L # in IV group. | Chan et al. (2005) [55] |
| Assessment of the effect of oral AA supplementation on AA concentration in plasma. (France) | Prospective, randomized, observational study, 33 HD patients (mean: age 52, HD vintage 6.1 years, session duration 4,2 h thrice a week, Kt/V 1.2) received 250 mg of AA orally after each HD for 2 months or no drug. AA in pre-HD plasma was determined with HPLC. | Mean AA-concentration increased 3.4 fold from the initial 19.5 ± 13.5 μmol/L in the supplemented group only. | Fumeron et al. (2005) [17] |
| Studying the effect of oral AA supplementation on AA concentration in serum. (Belgium) | Prospective observational, study. 92 HD patients (mean: age 67, HD vintage 2.9 years, Kt/V 1.4) AA was administered orally, for 3 months, after HD sessions at a dose of 360 or 1500 mg/week. Pre-HD AA concentration was determined with the enzymatic spectrophotometry. | Median AA concentration increased from 0.22 to 0.33 and to 0.63 mg/dl* after supplementation of 360 and 1500 mg/week, respectively. | De Vriese et al. (2007) [53] |
| Studying the effect of oral AA supplementation on concentration of AA in plasma. (Japan) | Prospective, observational study, 16 HD patients (mean: age 64, HD vintage 7.9 years, HD dose unknown). 1st month of the study: oral administration of 200 mg of AA 1 h before HD, thrice a week. 400 mg and 1000 mg were given during the 2nd and the 3rd month, respectively. AA in pre-HD plasma m was determined with HPLC. | Baseline mean AA concentration was 43.6 μmol/L and increased 2.1, 2.8 and 3.2 folds after consecutive doses of 200, 400, and 1000 mg respectively. | Washio et al.(2008) [19] |
| Studying the effect of oral AA supplementation on HD-patients with initially low AA level in plasma. (China) | Prospective, randomized, cross-over, observational study, 100 AA deficient HD patients (mean: age 64, HD vintage 48 months, Kt/V 1.5, initial AA concentration in plasma < 4 μg/mL *) received 200 mg/day of AA orally or no drug, for 3 months. AA was determined with HPLC in pre-HD plasma m. | AA concentration raised above 4 μg/mL ** in 80% of patients exceeding baseline value 4.5 to 7 folds. | Zhang et al. (2013) [54] |
| Studying the effects of intravenous AA supplementation on AA concentration in serum of children treated with HD. (Egypt) | Prospective, open label, randomized, placebo-controlled trial, 60 children (mean: age 9 years, HD vintage 2.9 years; HD dose 9–12 h/week, Kt/V ≥ 1.2) 30 patients received 250 mg of AA IV after each HD session for 12 weeks, or placebo. AA determination in pre-HD serum with HPLC. | Mean AA concentration increased 2.5 folds from initial 8.97 ± 4.38 μmol/L. It remained unchanged after placebo. | El Mashad et al.(2016) [56] |
6. Inhibitory Effects of Ascorbic Acid Supplementation on the Intensity of Oxidative Stress among Hemodialysis Patients
| Objective | Study Description | Main Results | References |
|---|---|---|---|
| Assessment of the influence of oral AA supplementation on peroxidative damage of lipids in erythrocytes’ membranes. | Prospective, open label, randomized, placebo-controlled trial, 34 HD patients (mean age 46, HD vintage unknown, dose 12 h/week) received orally 250 mg of AA/day for 90 days (n = 15) or placebo. MDA content in erythrocytes and their osmotic fragility was measured in pre-HD samples. | Together with the increase of plasma AA concentration osmotic fragility of erythrocytes decreased by 13%. MDA concentration decreased by 18%. No changes were observed after placebo. | Candan et al. (2002) [10] |
| Evaluation of the influence of intravenous AA supplementation on ROS production and DNA damage in lymphocytes. | Prospective, open label, randomized, placebo-controlled trial, 51 HD patients (mean: age 59, HD vintage 46 months, cellulose membranes, HD dose 12–13.5 h/week) received 300 mg of AA or saline (n = 26) after HD for 8 weeks. ROS production was measured with DCF luminescence, DNA damage with 8-OHdG content in lymphocytes isolated from pre-HD blood samples. | Spontaneous and PMA- stimulated ROS production became 5 and 2 fold smaller respectively with no changes after placebo. 8-OHdG content decreased by 18% and inversely correlated with the increase in AA concentration (r= −0.65), while directly with spontaneous and PMA-stimulated ROS production (r= 0.49 and 0.63 respectively). | Tarng et al. (2004) [21] |
| Studying the short and long term effects of IV infusion of AA during HD sessions on HD-related oxidative stress. | Prospective, observational, open label study, 20 HD patients in the intervention and 20 in the control group, Kt/V: 1.2–1.5, Polysynthane membranes, mean HD vintage 12 months. 1000 mg of AA was infused during each HD-session for 2 months. | AA suppressed HD-related ROS formation in blood by 86%, compared to no-intervention, inhibited the rise in H2O2 content in plasma by 60% and phosphatidylcholine hydroperoxide in plasma and erythrocytes by 45% and 35% respectively. | Yang et al. (2006) [57] |
| Studying the effect of oral AA supplementation on LDL oxidation. | Randomized, placebo-controlled, open label trial, 34 patients initiating HD (mean age 57, cellulose acetate membranes) received 1 g/d of AA or placebo for median of one year, the number of patients in each group is unknown. | Oxidized TBARS-LDL concentration increased more in the placebo group (by 64% of initial value) than in the AA group (52%). | Ramos et al. (2008) [61] |
| Studying the effect of intravenous AA supplementation on lipid peroxidation and PON1 activity. | Prospective Observational Study, 33 HD patients (mean: age 66, HD vintage 80 months, HD dose 12 h/week, membrane type unknown) received 500 mg of AA IV thrice a week for 6 months. PON1 activity and lipid hydroperoxides concentration in pre-HD plasma was measured. | PON1 activity increased by 60% of initial value, lipid hydroperoxides concentration decreased by 25%. | Ferretti et al. (2008) [58] |
| Studying the effects of oral AA supplementation on lipid peroxidation. | Randomized, placebo-controlled, double blinded trial, 42 HD patients (mean age 60, HD vintage 6 years, HD dose 12 h/week, membranes type unknown) received 250 mg of AA thrice a week for 12 weeks (n =21) or placebo. Measurements were performed in Pre-HD plasma. | MDA concentration decreased by 20% of initial value, while AA increased by 36%. MDA concentration did not change after placebo. | Abdollahzad et al. (2009) [60] |
| Assessment of the modification of ferric infusion-dependent oxidative stress by infusion of AA. | Prospective, randomized, open-label, crossover study, 13 HD patients (mean age: 58, HD vintage 74 months, Kt/V 1.6, ferritin 703 ng/mL, HD membranes unknown). 100 mg of ferric sucrose alone (IS group) or with 300 mg of AA (IS + C group) was infused in interdialytic day separated by 2 week wash-out period. PBMC were subsequently isolated. | PBMC from 13 patients in the IS group compared to 7 patients in the IS + C presented loss of mitochondrial membrane potential. | Conner et al. (2012) [63] |
| Studying the effect of oral AA supplementation on erythrocytes’ membrane proteins oxidation and plasma FRAP. | Prospective observational study, 11 HD patients (mean HD vintage 6 years, HD dose and membranes type unknown), 1000 mg of AA a day was administered enterally for 4 weeks. Pre-HD blood samples were collected. | Plasma FRAP increased by 12%. Total erythrocytes’ membrane protein carbonyls decreased by 63% of the initial value. | Ruskovska et al. (2015) [62] |
7. Inhibitory Effects of Ascorbic Acid Supplementation on the Intensity of Inflammation among Hemodialysis Patients
8. Unfavorable Effects of Ascorbic Acid Supplementation on Oxidative Stress and Inflammation in Hemodialysis Patients
| Objective | Study Description | Main Results | References |
|---|---|---|---|
| Assessment of the oxidative stress changes related to intravenous AA infusion. | Placebo-controlled, open label, randomized trial, 29 HD patients (mean: age 64, HD vintage unknown, Kt/V 1.4, Excebrane membranes). 300 mg of IV AA (n = 18) or saline was administered at the beginning of HD session. Blood samples were collected shortly before and after the injection for LucCL assay. | LucCl in the AA group was 16-fold higher than in placebo group, and correlated with ferritin concentration (r2 = 0.87). | Chen et al. (2003) [20] |
| Evaluation of the effect of oral AA supplementation on plasma and erythrocyte markers of oxidative stress. | Prospective, randomized, observational study, 33 HD patients (mean: age 52, HD vintage 6.1 years, Kt/V 1.2, cellulose diacetate and polysulfone membranes) received 250 mg of AA orally after each HD for 2 months or no drug. Plasma concentrations of DHA/AA, albumin, hs-CRP, protein carbonyls, erythrocyte GSH/GSSG. | Despite the elevation of AA concentration levels of measured markers remained unchanged. | Fumeron et al. (2005) [17] |
| Studying the influence of oral or IV AA supplementation on concentration of F2-isoprostanes in plasma of HD patients with hyperferritinemia | Prospective, randomized, parallel observational study, 21 HD patients (mean: age 56, HD vintage > 6 months, serum ferritin 632 μg/L, sessions thrice a week, URR 76%, HD membrane unknown) received 250 mg of AA orally or intravenously after HD sessions for 8 weeks. F2-isoprostanes were measured in pre-HD plasma. | Despite the elevation of AA concentration in plasma, no changes of F2-isoprostanes concentration were observed. | Chan et al. (2006) [78] |
| Evaluation of the modification of HD and ferric iron infusion-dependent oxidative stress by oral and intravenous AA. | Placebo-controlled, open-label, randomized trial, 20 AA deficient HD patients (mean age: 73, HD vintage 39 months, Kt/V 1.4, polysulphone membranes). 100 mg ferric sucrose was infused in AA deficient patients during HD, then 250 mg of AA a day for 2 weeks and 50 mg/day for the next 2 weeks was supplemented orally, and ferric sucrose or saline or AA or both were infused during HD and blood samples were taken. AA was measured with HPLC. | Ferric sucrose infusion induced equal increase in plasma TBARS in AA deficient or non-deficient patients. TBARS raised by 44% when iron was infused alone or by 47% when combined with AA. | Eiselt et al. (2006) [38] |
| Evaluation of the effects of oral administration of AA-containing antioxidant cocktail on plasma/serum concentration of markers and mediators of oxidative stress and inflammation. | Cross-sectional at baseline, longitudinal with 8-week follow-up, double-blinded, placebo-controlled trial, 37 HD patients (mean: age 52, HD vintage 54 months, HD dose unknown, cellulose acetate membranes); 20 treated every day with cocktail containing 250 mg of AA. Concentrations of f-2 isoprostane, protein carbonyls, CRP and IL-6 in pre-HD plasma was measured. | Concentration of f-2 isoprostane, protein carbonyls, CRP, and IL-6 remained unaffected after the treatment. | Kamgar et al. (2007) [77] |
| Studying the effect of oral AA supplementation on MDA concentration in plasma. | Prospective observational study. 92 HD patients (mean: age 67, HD vintage 2.9 years, Kt/V 1.4, cellulose diacetate membranes). AA was administered orally, after each HD session first at a dose of 360 then 1500 mg/week for 3 months. MDA was quantified in pre-HD plasma. | Mean MDA concentration increased from 1.5 ± 0.4 μmol/L to 1.6 ± 0.5 and 1.9 ± 0.5 μmol/L after 3 and 6 months respectively. Parallel rise of AA concentration is presented in Table 2. | De Vriese et al. (2007) [53] |
| Assessment of the effects of orally administered AA on plasma activity and concentration of Cu/Zn-SOD and its expression in leukocytes. | Prospective observational study 16 HD patients (mean: age 64, HD vintage 7.9 years, HD dose and membranes unknown). 1st month of the study: oral administration of 200 mg of AA 1 h before HD sessions. 400 mg and 1000 mg of AA were given during the 2nd and the 3rd month, respectively. The levels of Cu/Zn-SOD in pre-HD plasma and its mRNA expression in leukocytes were determined. | No changes in plasma Cu/Zn-SOD concentration and activity or its mRNA expression were observed. The parallel rise of AA concentration is presented in Table 2. | Washio et al. (2008) [19] |
| Assessment of the modification of iron infusion-dependent oxidative stress and inflammatory response by infusion of AA. | Prospective, randomized, open-label, crossover study, 13 HD patients (mean age: 58, HD vintage 74 months, Kt/V 1.6, ferritin 703 ng/mL). 100 mg ferric sucrose alone (IS group) or with 300 mg of AA (IS + C group) was infused in interdialytic day separated by 2 week wash-out period. Blood samples for IL-1, IL-10, TNF-α, F2-isoprostanes and PBMC for the assessment of intracellular O2−. and H2O2 generation were taken. | Concentrations of: IL-1, IL- 10, TNF-α, F2-isoprostanes rose 2.4, 1.4, 1.8, 1.2 fold respectively in the IS + C group but not in the IS group. O2−. generation increased 2.4 fold more in the IS + C group than in the IS. H2O2 generation did not differ between groups. | Conner et al. (2012) [63] |
| Studying the effect of oral AA supplementation on serum albumin concentration in HD patients with low initial AA level and high hs-CRP level. | Prospective, randomized, cross-over, observational study, 100 HD patients (mean: age 64, HD vintage 48 months, Kt/V 1.5, membrane type unknown), AA concentration in plasma < 4 μg/mL *. Equal groups received 200 mg/day of AA orally or nothing for 3 months. Measurements were made in pre-HD plasma. | Albumin concentration was not influenced by AA supplementation despite the rise of AA plasma level (Table 2). | Zhang et al. (2013) [54] |
| Studying the relation between serum AA concentration and markers of oxidative stress (AOPP, AGEs) and inflammation (CRP, IL-6) in HD patients on intravenous AA supplementation. | Cross-sectional study, 21 HD patients (mean: age 54, HD vintage 45 months, Kt/V 1.7, polysulfone membranes) received 100 mg of AA thrice a week. Concentrations of markers were measured in pre-HD serum, AA with spectrophotometry. | AGEs concentration showed a correlation with AA concentration (r = 0.46). Other parameters did not correlate with AA. | Marques de Mattos et al. (2014) [73] |
9. Safety of Ascorbic Acid in Hemodialysis Patients
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heaf, J. Current trends in European renal epidemiology. Clin. Kidney J. 2017, 10, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Granata, S.; Zaza, G.; Simone, S.; Villani, G.; Latorre, D.; Pontrelli, P.; Carella, M.; Schena, F.; Grandaliano, G.; Pertosa, G. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genom. 2009, 10, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Coaccioli, S.; Standoli, M.L.; Biondi, R.; Panaccione, A.; Landucci, P.; Del Giorno, R.; Paladini, A.; Standoli, M.; Puxeddu, A. Open comparison study of oxidative stress markers between patients with chronic renal failure in conservative therapy and patients in haemodialysis. Clin. Ter. 2010, 161, 435–439. [Google Scholar]
- Varan, H.; Dursun, B.; Dursun, E.; Ozben, T.; Suleymanlar, G. Acute effects of hemodialysis on oxidative stress parameters in chronic uremic patients: Comparison of two dialysis membranes. Int. J. Nephrol. Renovasc. Dis. 2010, 3, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Hörl, W.H.; Steinhauer, H.B.; Schollmeyer, P. Plasma levels of granulocyte elastase during hemodialysis: Effects of different dialyzer membranes. Kidney Int. 1985, 28, 791–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liakopoulos, V.; Roumeliotis, S.; Zarogiannis, S.; Eleftheriadis, T.; Mertens, P.R. Oxidative stress in hemodialysis: Causative mechanisms, clinical implications, and possible therapeutic interventions. Semin. Dial. 2019, 32, 58–71. [Google Scholar] [CrossRef]
- Heidari, B. C-reactive protein and other markers of inflammation in hemodialysis patients. Casp. J. Intern. Med. 2013, 4, 611–616. [Google Scholar]
- Candan, F.; Gültekin, F.; Candan, F. Effect of vitamin C and zinc on osmotic fragility and lipid peroxidation in zinc-deficient haemodialysis patients. Cell Biochem. Funct. 2002, 20, 95–98. [Google Scholar] [CrossRef]
- Li, D.; Mehta, J. Oxidized LDL, a critical factor in atherogenesis. Cardiovasc. Res. 2005, 68, 353–354. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- Delaney, S.; Jarem, D.A.; Volle, C.B.; Yennie, C.J. Chemical and biological consequences of oxidatively damaged guanine in DNA. Free Radic. Res. 2012, 46, 420–441. [Google Scholar] [CrossRef] [Green Version]
- Ghiadoni, L.; Cupisti, A.; Huang, Y.; Mattei, P.; Cardinal, H.; Favilla, S.; Rindi, P.; Barsotti, G.; Taddei, S.; Salvetti, A. Endothelial dysfunction and oxidative stress in chronic renal failure. J. Nephrol. 2004, 17, 512–519. [Google Scholar]
- Deicher, R.; Ziai, F.; Bieglmayer, C.; Schillinger, M.; Hörl, W.H. Low Total Vitamin C Plasma Level Is a Risk Factor for Cardiovascular Morbidity and Mortality in Hemodialysis Patients. J. Am. Soc. Nephrol. 2005, 16, 1811–1818. [Google Scholar] [CrossRef]
- Ceballos-Picot, I.; Witko-Sarsat, V.; Merad-Boudia, M.; Nguyen, A.T.; Thévenin, M.; Jaudon, M.C.; Zingraff, J.; Verger, C.; Jingers, P.; Descamps-Latscha, B. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic. Biol. Med. 1996, 21, 845–853. [Google Scholar] [CrossRef]
- Fumeron, C.; Nguyen-Khoa, T.; Saltiel, C.; Kebede, M.; Buisson, C.; Drüeke, T.B.; Lacour, B.; Massy, Z.A. Effects of oral vitamin C supplementation on oxidative stress and inflammation status in haemodialysis patients. Nephrol. Dial. Transplant. 2005, 20, 1874–1879. [Google Scholar] [CrossRef] [Green Version]
- Meister, A. Glutathione-ascorbic acid antioxidant system in animals. J. Biol. Chem. 1994, 269, 9397–9400. [Google Scholar] [CrossRef]
- Washio, K.; Inagaki, M.; Tsuji, M.; Morio, Y.; Akiyama, S.; Gotoh, H.; Gotoh, T.; Gotoh, Y.; Oguchi, K. Oral vitamin C supplementation in hemodialysis patients and its effect on the plasma level of oxidized ascorbic acid and Cu/Zn superoxide dismutase, an oxidative stress marker. Nephron Clin. Pract. 2008, 109, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-T.; Lin, Y.-F.; Yu, F.-C.; Kao, W.-Y.; Huang, W.-H.; Yan, H.-C. Effect of ascorbic acid administration in hemodialysis patients on in vitro oxidative stress parameters: Influence of serum ferritin levels. Am. J. Kidney Dis. 2003, 42, 158–166. [Google Scholar] [CrossRef]
- Tarng, D.-C.; Liu, T.-Y.; Huang, T.-P. Protective effect of vitamin C on 8-hydroxy-2′-deoxyguanosine level in peripheral blood lymphocytes of chronic hemodialysis patients. Kidney Int. 2004, 66, 820–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, M.; Padayatty, S.J.; Espey, M.G. Vitamin C: A Concentration-Function Approach Yields Pharmacology and Therapeutic Discoveries. Adv. Nutr. 2011, 2, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.; Wang, Y.; Padayatty, S.J.; Morrow, J. A new recommended dietary allowance of vitamin C for healthy young women. Proc. Natl. Acad. Sci. USA 2001, 98, 9842–9846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendich, A.; Machlin, L.J.; Scandurra, O.; Burton, G.W.; Wayner, D.D.M. The antioxidant role of vitamin C. Adv. Free Radic. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Berretta, M.; Quagliariello, V.; Maurea, N.; Di Francia, R.; Sharifi, S.; Facchini, G.; Rinaldi, L.; Piezzo, M.; Manuela, C.; Nunnari, G.; et al. Multiple Effects of Ascorbic Acid against Chronic Diseases: Updated Evidence from Preclinical and Clinical Studies. Antioxidants 2020, 9, 1182. [Google Scholar] [CrossRef]
- Oudemans-van Straaten, H.M.; Man, A.M.S.; de Waard, M.C. Vitamin C revisited. Crit. Care 2014, 18, 460. [Google Scholar] [CrossRef] [Green Version]
- Nowak, D.; Piasecka, G.; Antczak, A.; Pietras, T. Effect of ascorbic acid on hydroxyl radical generation by chemical, enzymatic and cellular systems. Importance for antioxidant prevention of pulmonary emphysema. Biomed. Biochim. Acta 1991, 50, 265–272. [Google Scholar]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic Metals, Ascorbate and Free Radicals: Combinations to Avoid. Radiat. Res. 1996, 145, 532. [Google Scholar] [CrossRef] [Green Version]
- Kaur, B.; Rowe, B.H.; Stovold, E. Vitamin C supplementation for asthma. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Hartel, C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine 2004, 27, 101–106. [Google Scholar] [CrossRef]
- Bowie, A.G.; O’Neill, L.A.J. Vitamin C Inhibits NF-κB Activation by TNF Via the Activation of p38 Mitogen-Activated Protein Kinase. J. Immunol. 2000, 165, 7180–7188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spoelstra-de Man, A.M.E.; Elbers, P.W.G.; Oudemans-Van Straaten, H.M. Vitamin C: Should we supplement? Curr. Opin. Crit. Care 2018, 24, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Dashti-Khavidaki, S.; Hajhossein Talasaz, A.; Tabeefar, H.; Hajimahmoodi, M.; Moghaddam, G.; Khalili, H.; Lessan-Pezeshki, M.; Jahanmardi, A. Plasma Vitamin C Concentrations in Patients on Routine Hemodialysis and its Relationship to Patients Morbidity and Mortality. Int. J. Vitam. Nutr. Res. 2011, 81, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Papastephanidis, C.; Agroyannis, B.; Tzanatos-Exarchou, H.; Orthopoulos, B.; Koutsicos, D.; Frangos-Plemenos, M.; Kallitsis, M.; Yatzidis, H. Re-evaluation of Ascorbic Acid Deficiency in Hemodialysed Patients. Int. J. Artif. Organs 1987, 10, 163–165. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Chen, D.; Lu, Y. Binding of ascorbic acid and α-tocopherol to bovine serum albumin: A comparative study. Mol. BioSyst. 2014, 10, 326–337. [Google Scholar] [CrossRef]
- Canavese, C.; Petrarulo, M.; Massarenti, P.; Berutti, S.; Fenoglio, R.; Pauletto, D.; Lanfranco, G.; Bergamo, D.; Sandri, L.; Marangella, M. Long-term, low-dose, intravenous vitamin C leads to plasma calcium oxalate supersaturation in hemodialysis patients. Am. J. Kidney Dis. 2005, 45, 540–549. [Google Scholar] [CrossRef]
- Eiselt, J.; Racek, J.; Opatrný, K., Jr.; Trefil, L.; Stehlík, P. The Effect of Intravenous Iron on Oxidative Stress in Hemodialysis Patients at Various Levels of Vitamin C. Blood Purif. 2006, 24, 531–537. [Google Scholar] [CrossRef]
- Lim, H.-S.; Kim, H.-S.; Kim, J.K.; Park, M.; Choi, S.J. Nutritional Status and Dietary Management According to Hemodialysis Duration. Clin. Nutr. Res. 2019, 8, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panchal, S.; Schneider, C.; Malhotra, K. Scurvy in a hemodialysis patient. Rare or ignored? Hemodial. Int. 2018, 22, S83–S87. [Google Scholar] [CrossRef] [Green Version]
- Massicotte-Azarniouch, D.; McLean, L.; Brown, P.A. Uremic leontiasis ossea due to secondary hyperparathyroidism complicated by vitamin C deficiency in a non-adherent chronic hemodialysis patient: A case report. Clin. Nephrol. Case Stud. 2019, 7, 54–59. [Google Scholar] [CrossRef]
- Deicher, R.; Ziai, F.; Habicht, A.; Bieglmayer, C.; Schillinger, M.; Horl, W.H. Vitamin C plasma level and response to erythropoietin in patients on maintenance haemodialysis. Nephrol. Dial. Transplant. 2004, 19, 2319–2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pincemail, J.; Vanbelle, S.; Degrune, F.; Cheramy-Bien, J.-P.; Charlier, C.; Chapelle, J.-P.; Giet, D.; Collette, G.; Albert, A.; Defraigne, J.-O. Lifestyle Behaviours and Plasma Vitamin C and β-Carotene Levels from the ELAN Population (Liège, Belgium). J. Nutr. Metab. 2011, 2011, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagel, A.F.; Albrecht, H.; Dauth, W.; Hagel, W.; Vitali, F.; Ganzleben, I.; Schultis, H.W.; Konturek, P.C.; Stein, J.; Neurath, M.F.; et al. Plasma concentrations of ascorbic acid in a cross section of the German population. J. Int. Med. Res. 2018, 46, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Richter, A.; Kuhlmann, M.K.; Seibert, E.; Kotanko, P.; Levin, N.W.; Handelman, G.J. Vitamin C deficiency and secondary hyperparathyroidism in chronic haemodialysis patients. Nephrol. Dial. Transplant. 2008, 23, 2058–2063. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Liu, L.; Cheng, X.; Dong, J.; Geng, Q.; Zuo, L. Low levels of vitamin C in dialysis patients is associated with decreased prealbumin and increased C-reactive protein. BMC Nephrol. 2011, 12, 18. [Google Scholar] [CrossRef] [Green Version]
- Raimann, J.G.; Abbas, S.R.; Liu, L.; Larive, B.; Beck, G.; Kotanko, P.; Levin, N.W.; Handelman, G. The effect of increased frequency of hemodialysis on vitamin C concentrations: An ancillary study of the randomized Frequent Hemodialysis Network (FHN) daily trial. BMC Nephrol. 2019, 20, 179. [Google Scholar] [CrossRef] [Green Version]
- Coveney, N.; Polkinghorne, K.R.; Linehan, L.; Corradini, A.M.; Kerr, P.G. Water-soluble vitamin levels in extended hours hemodialysis. Hemodial. Int. 2011. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.E. Ascorbate compartmentalization in the CNS. Neurotox. Res. 1999, 1, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Tarng, D.-C.; Wei, Y.-H.; Huang, T.-P.; Kuo, B.I.T.; Yang, W.-C. Intravenous ascorbic acid as an adjuvant therapy for recombinant erythropoietin in hemodialysis patients with hyperferritinemia. Kidney Int. 1999, 55, 2477–2486. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, S.S.; Perilloux, A.; Pereira, R.C.; Handelman, G.; Wesseling-Perry, K.; Salusky, I.B. Vitamin C overload may contribute to systemic oxalosis in children receiving dialysis. Pediatr. Nephrol. 2021, 36, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000; ISBN 978-0-309-06935-9.
- De Vriese, A.S.; Borrey, D.; Mahieu, E.; Claeys, I.; Stevens, L.; Vanhaeverbeke, A.; Roelens, M.; Langlois, M.R. Oral Vitamin C Administration Increases Lipid Peroxidation in Hemodialysis Patients. Nephron Clin. Pract. 2007, 108, c28–c34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, Y.; Cheng, X.; Liu, L.; Bai, W.; Guo, W.; Wu, L.; Zuo, L. Cross-over study of influence of oral vitamin C supplementation on inflammatory status in maintenance hemodialysis patients. BMC Nephrol. 2013, 14, 252. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.; Irish, A.; Dogra, G. Efficacy and safety of oral versus intravenous ascorbic acid for anaemia in haemodialysis patients. Nephrology 2005, 10, 336–340. [Google Scholar] [CrossRef] [PubMed]
- El Mashad, G.; ElSayed, H.; Nosair, N. Effect of vitamin C supplementation on lipid profile, serum uric acid, and ascorbic acid in children on hemodialysis. Saudi J. Kidney Dis. Transplant. 2016, 27, 1148. [Google Scholar] [CrossRef]
- Yang, C.-C.; Hsu, S.-P.; Wu, M.-S.; Hsu, S.-M.; Chien, C.-T. Effects of vitamin C infusion and vitamin E-coated membrane on hemodialysis-induced oxidative stress. Kidney Int. 2006, 69, 706–714. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, G.; Bacchetti, T.; Masciangelo, S.; Pallotta, G. Lipid peroxidation in hemodialysis patients: Effect of vitamin C supplementation. Clin. Biochem. 2008, 41, 381–386. [Google Scholar] [CrossRef]
- Zasowska-Nowak, A.; Nowak, P.J.; Bialasiewicz, P.; Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Markowski, J.; Rutkowski, K.P.; Nowak, D. Strawberries Added to the Usual Diet Suppress Fasting Plasma Paraoxonase Activity and Have a Weak Transient Decreasing Effect on Cholesterol Levels in Healthy Nonobese Subjects. J. Am. Coll. Nutr. 2016. [Google Scholar] [CrossRef]
- Abdollahzad, H.; Eghtesadi, S.; Nourmohammadi, I.; Khadem-Ansari, M.; Nejad-Gashti, H.; Esmaillzadeh, A. Effect of Vitamin C Supplementation on Oxidative Stress and Lipid Profiles in Hemodialysis Patients. Int. J. Vitam. Nutr. Res. 2009, 79, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Ramos, R.; Martínez-Castelao, A. Lipoperoxidation and hemodialysis. Metabolism 2008, 57, 1369–1374. [Google Scholar] [CrossRef]
- Ruskovska, T.; Bennett, S.J.; Brown, C.R.; Dimitrov, S.; Kamcev, N.; Griffiths, H.R. Ankyrin is the major oxidised protein in erythrocyte membranes from end-stage renal disease patients on chronic haemodialysis and oxidation is decreased by dialysis and vitamin C supplementation. Free Radic. Res. 2015, 49, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Conner, T.A.; McQuade, C.; Olp, J.; Pai, A.B. Effect of intravenous vitamin C on cytokine activation and oxidative stress in end-stage renal disease patients receiving intravenous iron sucrose. BioMetals 2012, 25, 961–969. [Google Scholar] [CrossRef]
- Rodríguez-Ayala, E.; Anderstam, B.; Suliman, M.E.; Seeberger, A.; Heimbürger, O.; Lindholm, B.; Stenvinkel, P. Enhanced RAGE-mediated NFκB stimulation in inflamed hemodialysis patients. Atherosclerosis 2005, 180, 333–340. [Google Scholar] [CrossRef]
- Samouilidou, E.; Grapsa, E.; Karpouza, A.; Lagouranis, A. Reactive Oxygen Metabolites: A Link between Oxidative Stress and Inflammation in Patients on Hemodialysis. Blood Purif. 2007, 25, 175–178. [Google Scholar] [CrossRef]
- Biniaz, V.; Sadeghi Shermeh, M.; Ebadi, A.; Tayebi, A.; Einollahi, B. Effect of Vitamin C Supplementation on C-reactive Protein Levels in Patients Undergoing Hemodialysis: A Randomized, Double Blind, Placebo-Controlled Study. Nephrourol. Mon. 2013, 6. [Google Scholar] [CrossRef] [Green Version]
- Ivanovich, P.; Chenoweth, D.E.; Schmidt, R.; Klinkmann, H.; Boxer, L.A.; Jacob, H.S.; Hammerschmidt, D.E. Symptoms and activation of granulocytes and complement with two dialysis membranes. Kidney Int. 1983, 24, 758–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baradari, A.G.; Zeydi, A.E.; Espahbodi, F.; Aarabi, M. The effect of intravenous vitamin C on the phosphorus level reduction in hemodialysis patients: A double blind randomized clinical trial. Med. Glas. 2012, 9, 37–41. [Google Scholar]
- Płóciniczak, A.; Dzięgielewska-Gęsiak, S.; Brożek, A.; Blacha, A.; Nowicki, M.; Formanowicz, D. High sensitivity C-reactive protein as a cardiovascular risk marker in independent community-living elderly persons. J. Biol. Regul. Homeost. Agents 2018, 32, 1199–1204. [Google Scholar] [PubMed]
- Keypour, H.; Silver, J.; Wilson, M.T.; Hamed, M.Y. Studies on the reactions of ferric iron with ascorbic acid. A study of solution chemistry using Mössbauer spectroscopy and stopped-flow techniques. Inorg. Chim. Acta 1986, 125, 97–106. [Google Scholar] [CrossRef]
- Duesterberg, C.K.; Cooper, W.J.; Waite, T.D. Fenton-Mediated Oxidation in the Presence and Absence of Oxygen. Environ. Sci. Technol. 2005, 39, 5052–5058. [Google Scholar] [CrossRef]
- Wiswedel, I. F2-Isoprostanes: Sensitive Biomarkers of Oxidative Stress In Vitro and In Vivo: A Gas Chromatography-Mass Spectrometric Approach. In Lipidomics; Humana Press: Totowa, NJ, USA, 2009; pp. 3–16. [Google Scholar]
- Marques de Mattos, A.; Afonso Jordão, A.; Abrão Cardeal da Costa, J.; Garcia Chiarello, P. Study of Protein Oxidative Stress, Antioxidant Vitamins and Inflammation in Patients Undergoing either Hemodialysis or Peritoneal Dialysis. Int. J. Vitam. Nutr. Res. 2014, 84, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Nemet, I.; Monnier, V.M. Vitamin C Degradation Products and Pathways in the Human Lens. J. Biol. Chem. 2011, 286, 37128–37136. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.; Bekker, P.; Tsimikas, S. Advanced Glycation End Products and Diabetic Cardiovascular Disease. Cardiol. Rev. 2012, 20, 177–183. [Google Scholar] [CrossRef]
- Otero, P.; Viana, M.; Herrera, E.; Bonet, B. Antioxidant and Prooxidant Effects of Ascorbic Acid, Dehydroascorbic Acid and Flavonoids on LDL Submitted to Different Degrees of Oxidation. Free Radic. Res. 1997, 27, 619–626. [Google Scholar] [CrossRef]
- Kamgar, M.; Zaldivar, F.; Vaziri, N.D.; Pahl, M.V. Antioxidant Therapy Does Not Ameliorate Oxidative Stress and Inflammation in Patients with End-Stage Renal Disease. J. Natl. Med. Assoc. 2009, 101, 336–344. [Google Scholar] [CrossRef]
- Chan, D.; Irish, A.; Croft, K.D.; Dogra, G. Effect of ascorbic acid supplementation on plasma isoprostanes in haemodialysis patients. Nephrol. Dial. Transplant. 2006, 21, 234–235. [Google Scholar] [CrossRef] [Green Version]
- Cross, J.M.; Donald, A.E.; Nuttall, S.L.; Deanfield, J.E.; Woolfson, R.G.; Macallister, R.J. Vitamin C improves resistance but not conduit artery endothelial function in patients with chronic renal failure. Kidney Int. 2003, 63, 1433–1442. [Google Scholar] [CrossRef] [Green Version]
- Ashor, A.W.; Brown, R.; Keenan, P.D.; Willis, N.D.; Siervo, M.; Mathers, J.C. Limited evidence for a beneficial effect of vitamin C supplementation on biomarkers of cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. Nutr. Res. 2019, 61, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Roumeliotis, S.; Bozikas, A.; Eleftheriadis, T.; Dounousi, E. Antioxidant Supplementation in Renal Replacement Therapy Patients: Is There Evidence? Oxid. Med. Cell. Longev. 2019, 2019, 9109473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, Y.P.; Weins, A.; Yeung, M.Y. Accelerated Oxalosis Contributing to Delayed Graft Function after Renal Transplantation. Case Rep. Transplant. 2019, 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Palsson, R.; Chandraker, A.K.; Curhan, G.C.; Rennke, H.G.; McMahon, G.M.; Waikar, S.S. The association of calcium oxalate deposition in kidney allografts with graft and patient survival. Nephrol. Dial. Transplant. 2020, 35, 888–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Objective | Study Description | Main Results | References |
|---|---|---|---|
| Studying the effect of intravenous AA supplementation on serum CRP concentration. | Randomized, double blinded, placebo-controlled trial, 58 HD patients (mean: age 60, HD vintage 30 months, 12 h of HD a week, polysulphone membranes) received 500 mg of AA IV after HD sessions, for 8 weeks (n = 29) or saline. Pre-HD serum was analyzed. | CRP concentration decreased by 34% remaining unchanged after placebo. | Baradari et al. (2012) [68] |
| Studying the effect of oral AA supplementation on plasma hs-CRP level in AA deficient patients. | Prospective, randomized, cross-over, observational study, 100 HD patients (mean: age 64, HD vintage 48 months, Kt/V 1.5, membrane type unknown), initial AA concentration in plasma < 4 μg/mL *. Equal groups received 200 mg/day of AA orally or no drug for 3 months. Pre-HD plasma was analyzed. | Hs-CRP concentration decreased by 28 to 49%. AA concentration in plasma raised (Table 2). | Zhang et al. (2013) [54] |
| Studying the effect of intravenous AA supplementation on CRP concentration in serum. | Randomized, placebo-controlled, double blinded trial, 141 HD patients (mean: age 61, HD vintage 40 months, HD sessions 3 × 3.8 h, membrane type unknown) Equal groups received 250 mg of AA IV after HD for 8 weeks or saline or nothing. It is unknown if serum CRP was measured pre- or post-HD session. | Median CRP concentration decreased by 36% in the AA group while it increased by 27% and 58% in the placebo and control group respectively. | Biniaz et al. (2014) [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaghouri, P.; Maalouf, N.; Peters, S.L.; Nowak, P.J.; Peczek, K.; Zasowska-Nowak, A.; Nowicki, M. Two Faces of Vitamin C in Hemodialysis Patients: Relation to Oxidative Stress and Inflammation. Nutrients 2021, 13, 791. https://doi.org/10.3390/nu13030791
Chaghouri P, Maalouf N, Peters SL, Nowak PJ, Peczek K, Zasowska-Nowak A, Nowicki M. Two Faces of Vitamin C in Hemodialysis Patients: Relation to Oxidative Stress and Inflammation. Nutrients. 2021; 13(3):791. https://doi.org/10.3390/nu13030791
Chicago/Turabian StyleChaghouri, Patrick, Nour Maalouf, Sophia Lorina Peters, Piotr Jan Nowak, Katarzyna Peczek, Anna Zasowska-Nowak, and Michal Nowicki. 2021. "Two Faces of Vitamin C in Hemodialysis Patients: Relation to Oxidative Stress and Inflammation" Nutrients 13, no. 3: 791. https://doi.org/10.3390/nu13030791