Effect of Varying Molecular Weight of Oat β-Glucan Taken just before Eating on Postprandial Glycemic Response in Healthy Humans
Abstract
:1. Introduction
2. Material and Methods
2.1. Subjects and Study Design
2.2. Procedures
2.3. Preparation of Test Meals and OBG Treatments
2.4. Measurement of OBG Viscosity and MW
2.5. Sample Size and Randomization
2.6. Data Management and Statistical Analysis
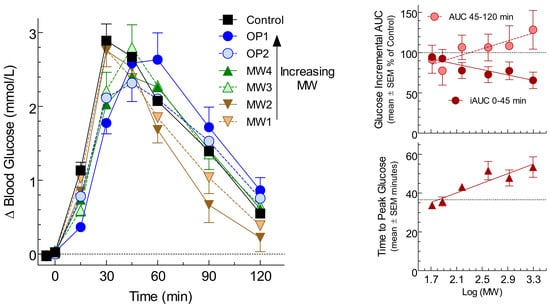
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- European Food Safety Authority (EFSA) Panel on dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction in post-prandial glycaemic response (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2207. [Google Scholar]
- Bureau of Nutritional Sciences, Food Directorate, Health Products and Food Branch, Health Canada. Draft guidance document on food health claims related to the reduction in post-prandial glycaemic response. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/public-involvement-partnerships/technical-consultation-draft-guidance-document-food-health-claims-related-post-prandial-glycaemia.html (accessed on 2 May 2020).
- Jenkins, D.J.A.; Wolever, T.M.S.; Taylor, R.H.; Barker, H.M.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.A.; Wolever, T.M.S.; Jenkins, A.L.; Thorne, M.J.; Lee, R.; Kalmusky, J.; Reichert, R.; Wong, G.S. The glycaemic index of foods tested in diabetic patients: A new basis for carbohydrate exchange favouring the use of legumes. Diabetologia 1983, 24, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosh, S.M.; Chu, Y. Systematic review of the effect of processing of whole-grain oat cereals on glycaemic responses. Br. J. Nutr. 2015, 114, 1256–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolever, T.M.S.; Katzman-Relle, L.; Jenkins, A.L.; Vuksan, V.; Josse, R.G.; Jenkins, D.J.A. Glycaemic index of 102 complex carbohydrate foods in patients with diabetes. Nutr. Res. 1994, 14, 651–669. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; van Klinken, B.J.W.; Spruill, S.E.; Jenkins, A.L.; Chu, Y.; Harkness, L. Effect of serving size and addition of sugar on the glycemic response elicited by oatmeal: A randomized, cross-over study. Clin. Nutr. ESPEN 2016, 16, 48–54. [Google Scholar] [CrossRef]
- Wood, P.J. Oat β-glucan: Structure, location and properties. In Oats: Chemistry and Technology; Webstger, F.H., Ed.; AACC Inc.: St Paul, MN, USA, 1986; pp. 121–152. [Google Scholar]
- Jenkins, D.J.A.; Wolever, T.M.S.; Leeds, A.R.; Gassull, M.A.; Haisman, P.; Dilawari, J.; Goff, D.V.; Metz, G.L.; Alberti, K.G.M. Dietary fibres, fibre analogues, and glucose tolerance: Importance of viscosity. Br. Med. J. 1978, 1, 1392–1394. [Google Scholar] [CrossRef] [Green Version]
- Wolever, T.M.S.; Jenkins, A.L.; Prudence, K.; Johnson, J.; Duss, R.; Chu, Y.; Steinert, R.E. Effect of adding oat bran to instant oatmeal on glycaemic response in humans—A study to establish the minimum effective dose of oat β-glucan. Food Funct. 2018, 9, 1692–1700. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.; Nineham, R.; Craddock, C.; Craig-McFeely, P.; Donaldson, K.; Leigh, T.; Snook, J. Fibre in diabetes. Lancet 1979, 1, 434–435. [Google Scholar] [CrossRef]
- Fuessl, S.; Adrian, T.E.; Bacarese-Hamilton, A.J.; Bloom, S.R. Guar in NIDD: Effect of different modes of administration on plasma glucose and insulin responses to a starch meal. Pract. Diabetes Int. 1986, 3, 258–260. [Google Scholar] [CrossRef]
- Steinert, R.E.; Raederstorff, D.; Wolever, T.M.S. Effect of consuming oat bran mixed in water before a meal on glycemic responses in healthy humans—A pilot study. Nutrients 2016, 8, 524. [Google Scholar] [CrossRef] [Green Version]
- Zurbriggen, B.Z.; Bailey, M.J.; Penttilä, M.E.; Poutanen, K.; Linko, M. Pilot scale production of a heterologous Trichoderma reesei cellulase by Saccharomyces cerevisiae. J. Biotechnol. 1990, 13, 267–278. [Google Scholar] [CrossRef]
- Aktas-Akyildiz, E.; Sibakov, J.; Nappa, M.; Hytönen, E.; Koksel, H.; Poutanen, K. Extraction of soluble b-glucan from oat and barley fractions: Process efficiency ad dispersion stability. J. Cereal Sci. 2018, 81, 60–68. [Google Scholar] [CrossRef]
- McCleary, B.V.; Mugford, D.C. Determination of β-glucan in barley and oats by streamlined enzymic method: Summary of collaborative study. J. AOAC Int. 1997, 80, 580–583. [Google Scholar] [CrossRef] [Green Version]
- Gamel, T.H.; Abdel-Aal, E.S.M.; Ames, N.P.; Henderson, K.; Prothon, F.; Kongraksawech, T.; Tosh, S.M. AACCI approved methods technical committee report: A new AACCI approved method (32-24.01) for measuring viscosity of β-glucan in cereal products using the rapid visco analyzer. Cereal Foods World 2015, 60, 279–283. [Google Scholar] [CrossRef]
- Gamel, T.H.; Abdel-Aal, E.S.M.; Ames, N.P.; Duss, R.; Tosh, S.M. Enzymatic extraction of beta-glucan from oat bran cereals and oat crackers and optimization of viscosity measurement. J. Cereal Sci. 2014, 59, 33–40. [Google Scholar] [CrossRef]
- Tosh, S.M.; Brummer, Y.; Miller, S.S.; Regand, A.; Defelice, C.; Duss, R.; Wolever, T.M.; Wood, P.J. Processing affects the physicochemical properties of β-glucan in oat bran cereal. J. Agric. Food Chem. 2010, 58, 7723–7730. [Google Scholar] [CrossRef]
- Brown, J.K.M. Experimental Design Generator and Randomizer. Available online: http://www.edgarweb.org.uk/ (accessed on 20 June 2019).
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980; p. 277. [Google Scholar]
- Wolever, T.M.S. Effect of blood sampling schedule and method calculating the area under the curve on validity and precision of glycaemic index value. Br. J. Nutr. 2004, 91, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Wood, P.J.; Beer, M.U.; Butler, G. Evaluation of the role of concentration and molecular weight of oat β-glucan in determining effect of viscosity on plasma glucose and insulin following an oral glucose load. Br. J. Nutr. 2000, 84, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Tosh, S.M.; Brummer, Y.; Wolever, T.M.S.; Wood, P.J. Glycemic response to oat bran muffins treated to vary molecular weight of β-glucan. Cereal Chem. 2008, 85, 211–217. [Google Scholar] [CrossRef]
- Kwong, M.G.; Wolever, T.M.S.; Brummer, Y.; Tosh, S.M. Increasing the viscosity of oat β-glucan beverages by reducing solution volume does not reduce glycaemic responses. Br. J. Nutr. 2013, 110, 1465–1471. [Google Scholar] [CrossRef] [Green Version]
- Marciani, L.; Gowland, P.A.; Spiller, R.C.; Manoj, P.; Moore, R.J.; Young, P.; Fillery-Travis, A.J. Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G1227–G1233. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.J.; Braaten, J.T.; Scott, F.W.; Riedel, K.D.; Wolynetz, M.S.; Collins, M.W. Effect of dose and modification of viscous properties of oat gum on plasma glucose and insulin following an oral glucose load. Br. J. Nutr. 1994, 72, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Biörklund, M.; van Rees, A.; Mensink, R.P.; Önning, G. Changes in serum lipids and postprandial glucose and insulin concentrations after consumption of beverages with β-glucans from oats or barley: A randomized dose-controlled trial. Eur. J. Clin. Nutr. 2005, 59, 1272–1281. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Ghani, M.A.; Williams, K.; DeFronzo, R.A.; Stern, M. What is the best predictor of future type 2 diabetes? Diabetes Care 2007, 30, 1544–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, D.B.; Ellis, P.R.; Baylis, J.M.; Fielden, P.; Ajodhia, J.; Leeds, A.R.; Jepson, E.M. Low dose guar in a novel food product: Improved metabolic control in non-insulin-dependent diabetes. Diabet. Med. 1987, 4, 111–115. [Google Scholar] [CrossRef] [PubMed]
- McGibbon, A.; Adams, L.; Ingersoll, K.; Kader, T.; Tugwell, B. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Glycemic Management of Adults with Type 1 Diabetes in Adults. Can. J. Diabetes 2018, 42 (Suppl. 1), S80–S87. [Google Scholar] [CrossRef] [Green Version]
- Luijf, Y.M.; van Bon, A.C.; Hoekstra, J.B.; DeVries, J.H. Premeal injection of rapid-acting insulin reduces postprandial glycemic excursions in type 1 diabetes. Diabetes Care 2010, 33, 2152–2155. [Google Scholar] [CrossRef] [Green Version]
- Thuillier, P.; Sonnet, E.; Alavi, Z.; Roudaut, N.; Nowak, E.; Dion, A.; Kerlan, V. Comparison between preprandial vs. postprandial insulin aspart in patients with type 1 diabetes in insulin pump and real-time continuous glucose monitoring. Diabetes Metab. Res. Rev. 2018, 34, e3019. [Google Scholar]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 2018, 31, 35–51. [Google Scholar] [CrossRef]
- McPhee, J.B.; Schertzer, J.D. Immunometabolism of obesity and diabetes: Microbiota link compart-mentalized immunity in the gut to metabolic tissue inflammation. Clin. Sci. 2015, 129, 1083–1096. [Google Scholar] [CrossRef]



| Preload | Amount (g) | Protein (g) | Fat (g) | Total Carb. (g) | Total Dietary Fiber (g) | Oat β-Glucan | |||
|---|---|---|---|---|---|---|---|---|---|
| Amount (g) | MW (kDa) | Viscosity (cP) | |||||||
| Initial | PD * | ||||||||
| Control | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - |
| MW1 | 7.48 | na | na | na | 5.0 | 4.0 | 52 ± 5 | 14 ± 4 | 10 ± 1 |
| MW2 | 7.41 | na | na | na | 5.1 | 4.0 | 76 ± 8 | 17 ± 3 | 14 ± 1 |
| MW3 | 8.28 | na | na | na | 5.7 | 4.0 | 153 ± 5 | 28 ± 2 | 31 ± 1 |
| MW4 | 10.42 | na | na | na | 5.1 | 4.0 | 393 ± 31 | 35 ± 5 | 40 ± 1 |
| OP1 | 13.7 | 3.2 | 0.7 | 8.4 | 7.2 | 4.0 | 1980 ± 265 | 25 ± 1 | 112 ± 9 |
| OP2 | 11.90 | 0.4 | 0.1 | 10.7 | 5.5 | 4.0 | 841 ± 110 | 117 ± 7 | 143 ± 7 |
| Preload | Palatability (mm) | Fasting Glucose (mmol/L) | Peak Rise (mmol/L) | Incremental Area under the Curve (mmol × min/L) | Time to Peak (min) | ||
|---|---|---|---|---|---|---|---|
| 0–45 min | 45–120 min | 0–120 min | |||||
| Control | 68 ± 6 | 4.39 ± 0.08 | 3.10 ± 0.27 | 80 ± 6 | 118 ± 15 | 198 ± 20 | 37 ± 3 |
| MW1 | 47 ± 7 * | 4.48 ± 0.07 | 2.89 ± 0.23 | 75 ± 6 | 98 ± 11 | 173 ± 15 | 34 ± 2 |
| MW2 | 46 ± 6 * | 4.38 ± 0.08 | 2.89 ± 0.23 | 73 ± 6 | 85 ± 11 | 159 ± 14 | 36 ± 2 |
| MW3 | 34 ± 7 * | 4.34 ± 0.06 | 2.93 ± 0.30 | 63 ± 6 * | 123 ± 16 | 186 ± 21 | 43 ± 2 |
| MW4 | 34 ± 8 * | 4.44 ± 0.08 | 2.70 ± 0.32 | 60 ± 7 * | 122 ± 17 | 181 ± 22 | 52 ± 5 * |
| OP1 | 43 ± 7 * | 4.47 ± 0.08 | 2.90 ± 0.31 | 52 ± 4 * | 143 ± 18 | 195 ± 21 | 53 ± 5 * |
| OP2 | 45 ± 7 * | 4.40 ± 0.07 | 2.55 ± 0.29 | 61 ± 5 * | 122 ± 18 | 183 ± 21 | 48 ± 4 |
| p ** | <0.0001 | 0.095 | 0.19 | <0.0001 | 0.002 | 0.20 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolever, T.M.S.; Mattila, O.; Rosa-Sibakov, N.; Tosh, S.M.; Jenkins, A.L.; Ezatagha, A.; Duss, R.; Steinert, R.E. Effect of Varying Molecular Weight of Oat β-Glucan Taken just before Eating on Postprandial Glycemic Response in Healthy Humans. Nutrients 2020, 12, 2275. https://doi.org/10.3390/nu12082275
Wolever TMS, Mattila O, Rosa-Sibakov N, Tosh SM, Jenkins AL, Ezatagha A, Duss R, Steinert RE. Effect of Varying Molecular Weight of Oat β-Glucan Taken just before Eating on Postprandial Glycemic Response in Healthy Humans. Nutrients. 2020; 12(8):2275. https://doi.org/10.3390/nu12082275
Chicago/Turabian StyleWolever, Thomas M. S., Outi Mattila, Natalia Rosa-Sibakov, Susan M. Tosh, Alexandra L. Jenkins, Adish Ezatagha, Ruedi Duss, and Robert E. Steinert. 2020. "Effect of Varying Molecular Weight of Oat β-Glucan Taken just before Eating on Postprandial Glycemic Response in Healthy Humans" Nutrients 12, no. 8: 2275. https://doi.org/10.3390/nu12082275