Chronic Supplementation with a Mix of Salvia officinalis and Salvia lavandulaefolia Improves Morris Water Maze Learning in Normal Adult C57Bl/6J Mice
Abstract
:1. Introduction
2. Materiel and Methods
2.1. Animals and Nutritional Supplementation
2.2. Behavioral Testing
2.2.1. Y-Maze
2.2.2. Spatial Learning and Reference Memory in the Morris Water Maze
Training Phase
Probe Test
2.3. Biochemical/Histological Measurements
2.3.1. Evaluation of Lipid Peroxidation (MDA Dosage) in Cortex
2.3.2. Measurement of Superoxide Dismutase Activity in Cortex in Red Blood Cells
2.3.3. Immunohistochemical Detection of Doublecortin (DCX)-Positive Cells
2.3.4. Immunohistochemical Detection of c-Fos Positive Cells
2.3.5. Measurement of Neurotrophins, Neurotrophin Receptor, CaM Kinase II and Glucocorticoid Receptors by Western Blot
2.4. Statistical Analysis
3. Results
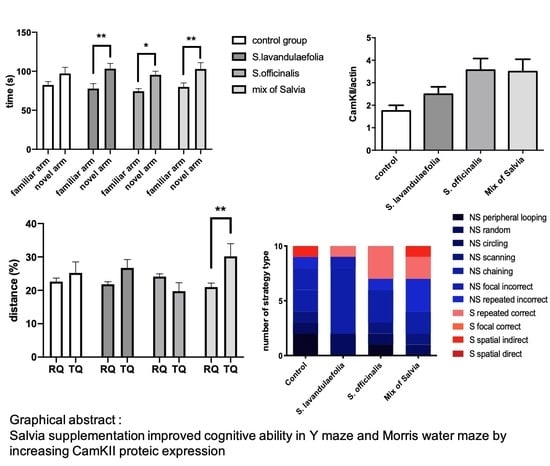
3.1. Acute Salvia Supplementation Improved Mice Cognitive Ability in Y-maze Test
3.2. Chronic mix of Salvia Supplementation Improved Cognitive Ability in Morris Water Maze
3.3. Chronic Mix of Salvia Supplementation Modulated Strategies during Spatial Memory
3.4. Chronic Mix of Salvia Supplementations Did Not Impact Oxidative Stress
3.5. Chronic Mix of Salvia Supplemention Did Not Impact Hippocampic Neurogenesis
3.6. Chronic Mix of Salvia Supplementation Did Not Impact Neuronal Activity
3.7. Chronic Mix of Salvia Supplementation Did Not Impact Neurotrophic Factor But Increased CaMKII Protein Expression
3.8. Chronic Mix of Salvia Supplementation Did Not Impact the Expression of Glucocorticoid Receptor and Its Phosphorylated Form in the Prefrontal Cortex of Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perry, E.K.; Pickering, A.T.; Wang, W.W.; Houghton, P.J.; Perry, N.S.L. Medicinal Plants and Alzheimer’s Disease: From Ethnobotany to Phytotherapy. J. Pharm. Pharmacol. 1999, 51, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Tildesley, N.; Kennedy, D.; Perry, E.; Ballard, C.; Savelev, S.; Wesnes, K.; Scholey, A. Salvia lavandulaefolia (Spanish Sage) enhances memory in healthy young volunteers. Pharmacol. Biochem. Behav. 2003, 75, 669–674. [Google Scholar] [CrossRef]
- Bernardini, S.; Tiezzi, A.; Masci, V.L.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2017, 32, 1926–1950. [Google Scholar] [CrossRef]
- Ovidi, E.; Masci, V.L. Salvia species, Interesting Plants Offering Perspectives in Alzheimer’s Disease. Curr. Tradit. Med. 2018, 4, 184–191. [Google Scholar] [CrossRef]
- Miroddi, M.; Navarra, M.; Quattropani, M.C.; Calapai, F.; Gangemi, S.; Calapai, G. Systematic Review of Clinical Trials Assessing Pharmacological Properties of Salvia Species on Memory, Cognitive Impairment and Alzheimer’s Disease. CNS Neurosci. Ther. 2014, 20, 485–495. [Google Scholar] [CrossRef]
- Eidi, M.; Eidi, A.; Bahar, M. Effects of Salvia officinalis L. (sage) leaves on memory retention and its interaction with the cholinergic system in rats. Nutrition 2006, 22, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Smach, M.; Hafsa, J.; Charfeddine, B.; Dridi, H.; Limem, K. Effects of sage extract on memory performance in mice and acetylcholinesterase activity. Ann. Pharm. Fr. 2015, 73, 281–288. [Google Scholar] [CrossRef]
- Hasanein, P.; Felehgari, Z.; Emamjomeh, A. Preventive effects of Salvia officinalis L. against learning and memory deficit induced by diabetes in rats: Possible hypoglycaemic and antioxidant mechanisms. Neurosci. Lett. 2016, 622, 72–77. [Google Scholar] [CrossRef]
- El-Kholy, W.M.; El-Habibi, E.M. Oxidative stress in brains of rats intoxicated with aluminum and the neuromodulating effect of different forms of sage. J. Am. Sci. 2010, 6, 12. [Google Scholar]
- Kishore, S.; Anitha, K.; Shireesha, N.; Pratima, K.; Ravi kumar, A.; Shobana, K. Evaluation of nootropic activity of salvia officinalis L.extract using different experimental models in rats. Glob. Trends Pharm. Sci. 2014, 5, 2. [Google Scholar]
- Mahmoodi, G.; Amini, S. The effect of Salvia officinalis hydroalcoholic extract on scopolamine-induced memory impairment in adult male mice. J. Basic Res. Med. Sci. 2019, 6, 8. [Google Scholar]
- Perry, N.S.L.; Houghton, P.J.; Theobald, A.; Jenner, P.; Perry, E.K. In-vitro Inhibition of Human Erythrocyte Acetylcholinesterase bySalvia lavandulaefoliaEssential Oil and Constituent Terpenes. J. Pharm. Pharmacol. 2000, 52, 895–902. [Google Scholar] [CrossRef]
- Savelev, S.; Okello, E.; Perry, N.; Wilkins, R.; Perry, E. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol. Biochem. Behav. 2003, 75, 661–668. [Google Scholar] [CrossRef]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Protective properties of Salvia lavandulifolia Vahl. essential oil against oxidative stress-induced neuronal injury. Food Chem. Toxicol. 2015, 80, 154–162. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Dodd, F.L.; Robertson, B.; Okello, E.J.; Reay, J.; Scholey, A.; Haskell, C.F. Monoterpenoid extract of sage (Salvia lavandulaefolia ) with cholinesterase inhibiting properties improves cognitive performance and mood in healthy adults. J. Psychopharmacol. 2010, 25, 1088–1100. [Google Scholar] [CrossRef]
- Tildesley, N.; Kennedy, D.; Perry, E.; Ballard, C.; Wesnes, K.; Scholey, A. Positive modulation of mood and cognitive performance following administration of acute doses of Salvia lavandulaefolia essential oil to healthy young volunteers. Physiol. Behav. 2005, 83, 699–709. [Google Scholar] [CrossRef]
- Scholey, A.; Tildesley, N.T.J.; Ballard, C.; Wesnes, K.; Tasker, A.; Perry, E.K.; Kennedy, D.O. An extract of Salvia (sage) with anticholinesterase properties improves memory and attention in healthy older volunteers. Psychopharmacology 2008, 198, 127–139. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2003, 28, 53–59. [Google Scholar] [CrossRef]
- Dinel, A.-L.; Joffre, C.; Trifilieff, P.; Aubert, A.; Foury, A.; Le Ruyet, P.; Layé, S. Inflammation early in life is a vulnerability factor for emotional behavior at adolescence and for lipopolysaccharide-induced spatial memory and neurogenesis alteration at adulthood. J. Neuroinflamm. 2014, 11, 155. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Maei, H.R.; Zaslavsky, K.; Teixeira, C.; Frankland, P.W. What is the Most Sensitive Measure of Water Maze Probe Test Performance? Front. Integr. Neurosci. 2009, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brody, D.L.; Holtzman, D.M. Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Exp. Neurol. 2005, 197, 330–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garthe, A.; Behr, J.; Kempermann, G. Adult-Generated Hippocampal Neurons Allow the Flexible Use of Spatially Precise Learning Strategies. PLoS ONE 2009, 4, e5464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, S.; Teixeira, C.; Zaslavsky, K.; Wheeler, A.L.; Wang, A.H.; Sakaguchi, M.; Lozano, A.M.; Frankland, P.W.; Martinez-Canabal, A. Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory. Hippocampus 2010, 21, 1348–1362. [Google Scholar] [CrossRef]
- Ruediger, S.; Spirig, D.; Donato, F.; Caroni, P. Goal-oriented searching mediated by ventral hippocampus early in trial-and-error learning. Nat. Neurosci. 2012, 15, 1563–1571. [Google Scholar] [CrossRef]
- Bensalem, J.; Servant, L.; Alfos, S.; Gaudout, D.; Layé, S.; Lafenêtre, P.; Pallet, V. Dietary Polyphenol Supplementation Prevents Alterations of Spatial Navigation in Middle-Aged Mice. Front. Behav. Neurosci. 2016, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Boitard, C.; Cavaroc, A.; Sauvant, J.; Aubert, A.; Castanon, N.; Layé, S.; Ferreira, G. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav. Immun. 2014, 40, 9–17. [Google Scholar] [CrossRef]
- Rincel, M.; Lepinay, A.L.; Janthakhin, Y.; Soudain, G.; Yvon, S.; Da Silva, S.; Joffre, C.; Aubert, A.; Sere, A.; Laye, S.; et al. Maternal high-fat diet and early life stress differentially modulate spine density and dendritic morphology in the medial prefrontal cortex of juvenile and adult rats. Brain Struct Funct 2018, 223, 883–895. [Google Scholar] [CrossRef]
- Dinel, A.; Rey, C.; Baudry, C.; Fressange-Mazda, C.; Le Ruyet, P.; Nadjar, A.; Pallet, P.; Joffre, C.; Layé, S. Enriched dairy fat matrix diet prevents early life lipopolysaccharide-induced spatial memory impairment at adulthood. Prostaglandins Leukot. Essent. Fat. Acids 2016, 113, 9–18. [Google Scholar] [CrossRef]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of Substances to Laboratory Animals: Routes of Administration and Factors to Consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Sandi, C. Stress and cognition. Wiley Interdiscip. Rev. Cogn. Sci. 2013, 4, 245–261. [Google Scholar] [CrossRef]
- Carew, T.J. Molecular Enhancement of Memory Formation. Neuron 1996, 16, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Kandel, E.R. The Molecular Biology of Memory Storage: A Dialog Between Genes and Synapses. Biosci. Rep. 2001, 21, 565–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sossin, W.S. Chapter 1 Molecular Memory Traces; Elsevier: Amsterdam, The Netherlands, 2008; Volume 169, pp. 3–25. [Google Scholar]
- Martin, K.C.; Barad, M.; Kandel, E.R. Local protein synthesis and its role in synapse-specific plasticity. Curr. Opin. Neurobiol. 2000, 10, 587–592. [Google Scholar] [CrossRef]
- Kelleher, R.J.; Govindarajan, A.; Tonegawa, S. Translational Regulatory Mechanisms in Persistent Forms of Synaptic Plasticity. Neuron 2004, 44, 59–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bramham, C.; Wells, D.G. Dendritic mRNA: Transport, translation and function. Nat. Rev. Neurosci. 2007, 8, 776–789. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.M.; Kater, S.B. Dendritic Spines: Cellular Specializations Imparting Both Stability and Flexibility to Synaptic Function. Annu. Rev. Neurosci. 1994, 17, 341–371. [Google Scholar] [CrossRef]
- Impey, S.; Mark, M.; Villacres, E.C.; Poser, S.; Chavkin, C.; Storm, D.R. Induction of CRE-Mediated Gene Expression by Stimuli That Generate Long-Lasting LTP in Area CA1 of the Hippocampus. Neuron 1996, 16, 973–982. [Google Scholar] [CrossRef]
- Impey, S.; Smith, D.M.; Obrietan, K.; Donahue, R.; Wade, C.; Storm, D.R. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat. Neurosci. 1998, 1, 595–601. [Google Scholar] [CrossRef]
- Impey, S.; McCorkle, S.R.; Cha-Molstad, H.; Dwyer, J.M.; Yochum, G.S.; Boss, J.M.; McWeeney, S.K.; Dunn, J.J.; Mandel, G.; Goodman, R.H. Defining the CREB Regulon. Cell 2004, 119, 1041–1054. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.A.; Impey, S.; Storm, D.R.; Stryker, M.P. CRE-Mediated Gene Transcription in Neocortical Neuronal Plasticity during the Developmental Critical Period. Neuron 1999, 22, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Barco, A.; Bailey, C.H.; Kandel, E.R. Common molecular mechanisms in explicit and implicit memory. J. Neurochem. 2006, 97, 1520–1533. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Sabatini, B.L. Anatomical and Physiological Plasticity of Dendritic Spines. Annu. Rev. Neurosci. 2007, 30, 79–97. [Google Scholar] [CrossRef] [Green Version]
- Sairanen, M.; Lucas, G.; Ernfors, P.; Castrén, M.L.; Castren, E. Brain-Derived Neurotrophic Factor and Antidepressant Drugs Have Different But Coordinated Effects on Neuronal Turnover, Proliferation, and Survival in the Adult Dentate Gyrus. J. Neurosci. 2005, 25, 1089–1094. [Google Scholar] [CrossRef] [Green Version]
- Scharfman, H.E.; Goodman, J.; MacLeod, A.; Phani, S.; Antonelli, C.; Croll, S.D. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 2005, 192, 348–356. [Google Scholar] [CrossRef]
- Henry, R.A.; Hughes, S.; Connor, B. AAV-mediated delivery of BDNF augments neurogenesis in the normal and quinolinic acid-lesioned adult rat brain. Eur. J. Neurosci. 2007, 25, 3513–3525. [Google Scholar] [CrossRef]
- Gould, E.; Beylin, A.; Tanapat, P.; Reeves, A.; Shors, T.J. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 1999, 2, 260–265. [Google Scholar] [CrossRef]
- Altman, J. Are New Neurons Formed in the Brains of Adult Mammals? Science 1962, 135, 1127–1128. [Google Scholar] [CrossRef] [Green Version]
- Gross, C.G. Neurogenesis in the adult brain: Death of a dogma. Nat. Rev. Neurosci. 2000, 1, 67–73. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Kempermann, G.; Kuhn, H.-G.; Gage, F.H. More hippocampal neurons in adult mice living in an enriched environment. Nature 1997, 386, 493–495. [Google Scholar] [CrossRef]
- Kempermann, G.; Kuhn, H.-G.; Gage, F.H. Experience-Induced Neurogenesis in the Senescent Dentate Gyrus. J. Neurosci. 1998, 18, 3206–3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef] [Green Version]
- Van Praag, H.; Kempermann, G.; Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999, 2, 266–270. [Google Scholar] [CrossRef]
- He, J.; Yamada, K.; Nabeshima, T. A Role of Fos Expression in the CA3 Region of the Hippocampus in Spatial Memory Formation in Rats. Neuropsychopharmacology 2002, 26, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Colombo, D.; Serino, S.; Tuena, C.; Pedroli, E.; Dakanalis, A.; Cipresso, P.; Riva, G. Egocentric and allocentric spatial reference frames in aging: A systematic review. Neurosci. Biobehav. Rev. 2017, 80, 605–621. [Google Scholar] [CrossRef]
- Avraamides, M.N.; Kelly, J.W. Multiple systems of spatial memory and action. Cogn. Process. 2007, 9, 93–106. [Google Scholar] [CrossRef]
- Garthe, A.; Kempermann, G. An old test for new neurons: Refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front. Mol. Neurosci. 2013, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Dupret, D.; Revest, J.-M.; Koehl, M.; Ichas, F.; De Giorgi, F.; Costet, P.; Abrous, N.; Piazza, P.V. Spatial Relational Memory Requires Hippocampal Adult Neurogenesis. PLoS ONE 2008, 3, e1959. [Google Scholar] [CrossRef] [Green Version]
- Morris, R.G.M.; Garrud, P.; Rawlins, J.N.P.; O’Keefe, J. Place navigation impaired in rats with hippocampal lesions. Nature 1982, 297, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Guzowski, J.F.; Setlow, B.; Wagner, E.K.; McGaugh, J.L. Experience-Dependent Gene Expression in the Rat Hippocampus after Spatial Learning: A Comparison of the Immediate-Early GenesArc, c-fos, and zif268. J. Neurosci. 2001, 21, 5089–5098. [Google Scholar] [CrossRef] [Green Version]
- Bertaina, V.; Destrade, C. Differential time courses of c-fos mRNA expression in hippocampal subfields following acquisition and recall testing in mice. Cogn. Brain Res. 1995, 2, 269–275. [Google Scholar] [CrossRef]
- Vann, S.D.; Brown, M.W.; Erichsen, J.T.; Aggleton, J.P. Fos Imaging Reveals Differential Patterns of Hippocampal and Parahippocampal Subfield Activation in Rats in Response to Different Spatial Memory Tests. J. Neurosci. 2000, 20, 2711–2718. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; McQuade, J.M.S.; Vorhees, C.V.; Xu, M. Hippocampal expression of c-fos is not essential for spatial learning. Synapse 2002, 46, 91–99. [Google Scholar] [CrossRef]
- Shires, K.L.; Aggleton, J.P. Mapping immediate-early gene activity in the rat after place learning in a water-maze: The importance of matched control conditions. Eur. J. Neurosci. 2008, 28, 982–996. [Google Scholar] [CrossRef]
- Dong, M.; Wu, Y.; Fan, Y.; Xu, M.; Zhang, J. c-fos modulates brain-derived neurotrophic factor mRNA expression in mouse hippocampal CA3 and dentate gyrus neurons. Neurosci. Lett. 2006, 400, 177–180. [Google Scholar] [CrossRef]
- Boitard, C.; Etchamendy, N.; Sauvant, J.; Aubert, A.; Tronel, S.; Marighetto, A.; Layé, S.; Ferreira, G. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus 2012, 22, 2095–2100. [Google Scholar] [CrossRef]
- Yamauchi, T. Neuronal Ca2+/Calmodulin-Dependent Protein Kinase II—Discovery, Progress in a Quarter of a Century, and Perspective: Implication for Learning and Memory. Biol. Pharm. Bull. 2005, 28, 1342–1354. [Google Scholar] [CrossRef] [Green Version]
- Lisman, J.; Schulman, H.; Cline, H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002, 3, 175–190. [Google Scholar] [CrossRef]
- Zalcman, G.; Federman, N.; Romano, A. CaMKII Isoforms in Learning and Memory: Localization and Function. Front. Mol. Neurosci. 2018, 11, 445. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinel, A.-L.; Lucas, C.; Guillemet, D.; Layé, S.; Pallet, V.; Joffre, C. Chronic Supplementation with a Mix of Salvia officinalis and Salvia lavandulaefolia Improves Morris Water Maze Learning in Normal Adult C57Bl/6J Mice. Nutrients 2020, 12, 1777. https://doi.org/10.3390/nu12061777
Dinel A-L, Lucas C, Guillemet D, Layé S, Pallet V, Joffre C. Chronic Supplementation with a Mix of Salvia officinalis and Salvia lavandulaefolia Improves Morris Water Maze Learning in Normal Adult C57Bl/6J Mice. Nutrients. 2020; 12(6):1777. https://doi.org/10.3390/nu12061777
Chicago/Turabian StyleDinel, Anne-Laure, Céline Lucas, Damien Guillemet, Sophie Layé, Véronique Pallet, and Corinne Joffre. 2020. "Chronic Supplementation with a Mix of Salvia officinalis and Salvia lavandulaefolia Improves Morris Water Maze Learning in Normal Adult C57Bl/6J Mice" Nutrients 12, no. 6: 1777. https://doi.org/10.3390/nu12061777