Capsosiphon fulvescens Glycoproteins Enhance Probiotics-Induced Cognitive Improvement in Aged Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of L. plantarum
2.2. Isolation of Cf-hGP
2.3. Scheme for Oral Administration
2.4. Preparation of Crude Synaptosomal Fraction
2.5. Western Blot and Co-Immunoprecipitation
2.6. Double Immunofluorescence Staining
2.7. Rat Surgery and Microinjection
2.8. Morris Water Maze Test for Spatial Learning and Memory
2.9. Statistical Analysis
3. Results
3.1. Co-Treatment with Cf-hGP and L. plantarum Synergistically Improves Spatial Memory in Aged Rats
3.2. Synergistic Upregulation of Mature BDNF and Nrf2 Phosphorylation in the Dorsal Hippocampus by Cf-hGP Co-Treatment with L. plantarum is Accompanied by Downregulation of JNK Phosphorylation
3.3. Blockade of BDNF Signaling Overturns Increases in Nrf2 Phosphorylation and Decreases in JNK Phosphorylation, While Inhibition of Nrf2 Signaling Downregulates Decreases in JNK Phosphorylation and ER Stress Response to Co-Treatment
3.4. Blockade of BDNF Signaling or Inhibition of PP2A Overturned Decrease in eEF2 Phosphorylation by Co-Treatment with Cf-hGP and L. plantarum
3.5. Co-Treatment with Cf-hGP and L. plantarum Reduces Interaction between eEF2K and JNK
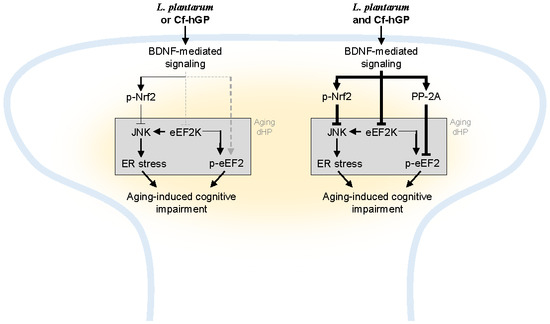
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rai, K.S.; Hattiangady, B.; Shetty, A.K. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur. J. Neurosci. 2007, 26, 1765–1779. [Google Scholar] [CrossRef]
- Olariu, A.; Cleaver, K.M.; Cameron, H.A. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J. Comp. Neurol. 2007, 501, 659–667. [Google Scholar] [CrossRef]
- Hattiangady, B.; Shetty, A.K. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol. Aging 2008, 29, 129–147. [Google Scholar] [CrossRef] [Green Version]
- Pruessner, J.C.; Collins, D.L.; Pruessner, M.; Evans, A.C. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. J. Neurosci. 2001, 21, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Kadar, T.; Sirimanne, E.; MacGibbon, A.; Guan, J. Age-related memory decline is associated with vascular and microglial degeneration in aged rats. Behav. Brain Res. 2012, 235, 210–217. [Google Scholar] [CrossRef]
- Leung, K.; Thuret, S. Gut microbiota: A modulator of brain. plasticity and cognitive function in ageing. Healthcare (Basel) 2015, 3, 898–916. [Google Scholar] [CrossRef] [Green Version]
- Hotel, A.C.P.; Cordoba, A. Health and nutritional properties of probiotics in food including powder milk with live Lactic acid bacteria. Prevention 2001, 5, 1–10. [Google Scholar]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Liu, W.H.; Wu, C.C.; Juan, Y.C.; Wu, Y.C.; Tsai, H.P.; Wang, S.; Tsai, Y.C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naive adult mice. Brain Res. 2016, 1631, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, P.T.; Lu, B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: Role of secreted proteins tPA and BDNF. Ageing Res. Rev. 2004, 3, 407–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Angelucci, A.; Costantin, L.; Braschi, C.; Mazzantini, M.; Babbini, F.; Fabbri, M.E.; Tessarollo, L.; Maffei, L.; Berardi, N.; et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur. J. Neurosci. 2006, 24, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef] [Green Version]
- Diógenes, M.J.; Costenla, A.R.; Lopes, L.V.; Jerónimo-Santos, A.; Sousa, V.C.; Fontinha, B.M.; Ribeiro, J.A.; Sebastião, A.M. Enhancement of LTP in aged rats is dependent on endogenous BDNF. Neuropsychopharmacology 2011, 36, 1823–1836. [Google Scholar] [CrossRef] [Green Version]
- Erickson, K.I.; Miller, D.L.; Roecklein, K.A. The aging hippocampus: Interactions between exercise, depression, and BDNF. Neuroscientist 2012, 18, 82–97. [Google Scholar] [CrossRef]
- Petzold, A.; Psotta, L.; Brigadski, T.; Endres, T.; Lessmann, V. Chronic BDNF deficiency leads to an age-dependent impairment in spatial learning. Neurobiol. Learn. Mem. 2015, 120, 52–60. [Google Scholar] [CrossRef]
- Taha, E.; Gildish, I.; Gal-Ben-Ari, S.; Rosenblum, K. The role of eEF2 pathway in learning and synaptic plasticity. Neurobiol. Learn. Mem. 2013, 105, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, B.M.; Pincus, D.; Gotthardt, K.; Gallagher, C.M.; Walter, P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect. Biol. 2013, 5, a013169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darling, N.J.; Cook, S.J. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta 2014, 1843, 2150–2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hetz, C.; Mollereau, B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat. Rev. Neurosci. 2014, 15, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Gavilán, M.P.; Pintado, C.; Gavilán, E.; Jiménez, S.; Ríos, R.M.; Vitorica, J.; Castaño, A.; Ruano, D. Dysfunction of the unfolded protein response increases neurodegeneration in aged rat hippocampus following proteasome inhibition. Aging Cell 2009, 8, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Duran-Aniotz, C.; Cabral-Miranda, F.; Vivar, J.P.; Hetz, C. Endoplasmic reticulum proteostasis impairment in aging. Aging Cell 2017, 16, 615–623. [Google Scholar] [CrossRef]
- Lee, A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 2005, 35, 373–381. [Google Scholar] [CrossRef]
- Borsello, T.; Clarke, P.G.; Hirt, L.; Vercelli, A.; Repici, M.; Schorderet, D.F.; Bogousslavsky, J.; Bonny, C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 2003, 9, 1180–1186. [Google Scholar] [CrossRef]
- Mazzitelli, S.; Xu, P.; Ferrer, I.; Davis, R.J.; Tournier, C. The loss of c-Jun N-terminal protein kinase activity prevents the amyloidogenic cleavage of amyloid precursor protein and the formation of amyloid plaques in vivo. J. Neurosci. 2011, 31, 16969–16976. [Google Scholar] [CrossRef]
- Bruna, B.; Lobos, P.; Herrera-Molina, R.; Hidalgo, C.; Paula-Lima, A.; Adasme, T. The signaling pathways underlying BDNF-induced Nrf2 hippocampal nuclear translocation involve ROS, RyR-mediated Ca2+ signals, ERK and PI3K. Biochem. Biophys. Res. Commun. 2018, 505, 201–207. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Choi, Y.H.; Lee, J.I.; Kim, I.H.; Nam, T.J. Antioxidant activity of oxygen evolving enhancer protein 1 purified from Capsosiphon fulvescens. J. Food Sci. 2015, 80, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kim, I.H.; Nam, T.J. Inhibition of AGS human gastric cancer cell invasion and proliferation by Capsosiphon fulvescens glycoprotein. Mol. Med. Rep. 2013, 8, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, M.J.; Nam, T.J. A polysaccharide of the marine alga Capsosiphon fulvescens induces apoptosis in AGS gastric cancer cells via an IGF-IR-mediated PI3K/Akt pathway. Cell Biol. Int. 2007, 31, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Ko, D.O.; Kim, B.S.; Ham, K.S.; Kang, S.G. Beneficial effect of seaweed on high-fat diet-induced oxidative stress and insulin resistance in rats. Food Sci. Biotechnol. 2015, 24, 2185–2191. [Google Scholar] [CrossRef]
- Kim, E.Y.; Nam, T.J.; Oh, J.H. Hydrophilic compartments of Capsosiphon fulvescens protein alleviate impaired spatial memory by regulating BDNF-mediated ER stress against chronic ethanol exposure. J. Funct. Foods 2017, 35, 474–480. [Google Scholar] [CrossRef]
- Oh, J.H.; Nam, T.J. Hydrophilic glycoproteins of an edible green alga Capsosiphon fulvescens prevent aging-induced spatial memory impairment by suppressing GSK-3β-mediated ER stress in dorsal hippocampus. Mar. Drugs 2019, 17, 168. [Google Scholar] [CrossRef] [Green Version]
- Wirths, O. Preparation of crude synaptosomal fractions from mouse brains and spinal cords. Bioprotocol 2017, 7, e2423. [Google Scholar] [CrossRef] [Green Version]
- Mota, S.I.; Costa, R.O.; Ferreira, I.L.; Santana, I.; Caldeira, G.L.; Padovano, C.; Fonseca, A.C.; Baldeiras, I.; Cunha, C.; Letra, L.; et al. Oxidative stress involving changes in Nrf2 and ER stress in early stages of Alzheimer’s disease. Biochim. Biophys. Acta 2015, 1852, 1428–1441. [Google Scholar] [CrossRef] [Green Version]
- Montanaro, L.; Sperti, S.; Testoni, G.; Mattioli, A. Effect of elongation factor 2 and of adenosine diphosphate-ribosylated elongation factor 2 on translocation. Biochem. J. 1976, 156, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Dinan, T.G.; Stilling, R.M.; Stanton, C.; Cryan, J.F. Collective unconscious: How gut microbes shape human behavior. J. Psychiatr. Res. 2015, 63, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulyaeva, N.V. Functional neurochemistry of the ventral and dorsal hippocampus: Stress, depression, dementia and remote hippocampal damage. Neurochem. Res. 2019, 44, 1306–1322. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. Proteostasis and aging. Nat. Med. 2015, 21, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; Huang, W.; Costa-Mattioli, M. Translational control in synaptic plasticity and cognitive dysfunction. Annu. Rev. Neurosci. 2014, 37, 17–38. [Google Scholar] [CrossRef] [Green Version]
- Costa-Mattioli, M.; Sossin, W.S.; Klann, E.; Sonenberg, N. Translational control of long-lasting synaptic plasticity and memory. Neuron 2009, 61, 10–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heise, C.; Gardoni, F.; Culotta, L.; di Luca, M.; Verpelli, C.; Sala, C. Elongation factor-2 phosphorylation in dendrites and the regulation of dendritic mRNA translation in neurons. Front. Cell. Neurosci. 2014, 8, 35. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.H.; Nam, T.-J.; Choi, Y.H. Capsosiphon fulvescens Glycoproteins Enhance Probiotics-Induced Cognitive Improvement in Aged Rats. Nutrients 2020, 12, 837. https://doi.org/10.3390/nu12030837
Oh JH, Nam T-J, Choi YH. Capsosiphon fulvescens Glycoproteins Enhance Probiotics-Induced Cognitive Improvement in Aged Rats. Nutrients. 2020; 12(3):837. https://doi.org/10.3390/nu12030837
Chicago/Turabian StyleOh, Jeong Hwan, Taek-Jeong Nam, and Youn Hee Choi. 2020. "Capsosiphon fulvescens Glycoproteins Enhance Probiotics-Induced Cognitive Improvement in Aged Rats" Nutrients 12, no. 3: 837. https://doi.org/10.3390/nu12030837