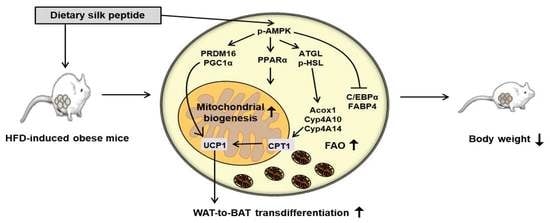
Dietary Silk Peptide Prevents High-Fat Diet-Induced Obesity and Promotes Adipose Browning by Activating AMP-Activated Protein Kinase in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of SP from Bombyx Mori
2.2. Cell Culture and Treatment
2.3. Animals and Diet
2.4. Rectal Temperature Measurement
2.5. Serum Biochemistry
2.6. Histological Analysis and Immunofluorescence Staining
2.7. Oil Red O Staining
2.8. Western Blot Analysis
2.9. Quantitative Reverse Transcription Polymerase Chain Reaction Analysis
2.10. Statistical Analysis
3. Results
3.1. Nutritional Analysis of the Silk Peptide
3.2. SP Inhibits Body Mass Gain in HFD-Induced Obese Mice
3.3. SP Reduces WAT Depot Size and Downregulates Adipogenic Gene Expression
3.4. SP Induces FAO in WAT
3.5. SP Promotes Browning in WAT
3.6. SP Ameliorates Hepatic Lipid Accumulation and Increases FAO in the Liver
3.7. SP Inhibits Lipid Accumulation and Increases the Expression of Markers of Browning in Primary sWAT-Derived Adipocytes
3.8. SP Reduces Lipid Drople Size and Increases UCP1 Expression by Inducing AMPK Phosphorylation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SP | silk peptide |
| WAT | white adipose tissue |
| BAT | brown adipose tissue |
| sWAT | subcutaneous adipose white tissue |
| vWAT | visceral white adipose tissue |
| FAO | fatty acid oxidation |
| ATGL | adipose triglyceride lipase |
| FABP4 | fatty acid-binding protein 4 |
| C/EBPα | CCAAT enhancer-binding protein α |
| DGAT1 | diacylglycerol acyltransferase 1 |
| AMPK | AMP-activated protein kinase |
| HSL | hormone-sensitive lipase |
| Cyp4a10 | cytochrome P450 4a10 |
| Cyp4a14 | cytochrome P450 4a14 |
| Acox1 | acyl-CoA oxidase 1 |
| CPT1 | carnitine palmitoyltransferase 1 |
| PPARα | peroxisome proliferator-activated receptor alpha |
| PRDM16 | PR domain-containing 16 |
| PGC1α | peroxisome proliferator-activated receptor γ coactivator 1α |
| SREBP1c | sterol regulatory element-binding transcription factor 1 |
| UCP1 | uncoupling protein 1 |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
References
- Romieu, I.; Dossus, L.; Barquera, S.; Blottiere, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martin-Cabrejas, M.A.; de Mejia, E.G. Cocoa Shell Aqueous Phenolic Extract Preserves Mitochondrial Function and Insulin Sensitivity by Attenuating Inflammation between Macrophages and Adipocytes In Vitro. Mol. Nutr. Food Res. 2019, 63, e1801413. [Google Scholar] [CrossRef]
- Calderon-Dominguez, M.; Sebastian, D.; Fucho, R.; Weber, M.; Mir, J.F.; Garcia-Casarrubios, E.; Obregon, M.J.; Zorzano, A.; Valverde, A.M.; Serra, D.; et al. Carnitine Palmitoyltransferase 1 Increases Lipolysis, UCP1 Protein Expression and Mitochondrial Activity in Brown Adipocytes. PLoS ONE 2016, 11, e0159399. [Google Scholar] [CrossRef]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy balance and obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Mottillo, E.P.; Granneman, J.G. Adipose tissue plasticity from WAT to BAT and in between. Biochim. Biophys. Acta 2014, 1842, 358–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Giralt, M.; Villarroya, F. White, brown, beige/brite: Different adipose cells for different functions? Endocrinology 2013, 154, 2992–3000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, P.; Spiegelman, B.M. Cell biology of fat storage. Mol. Biol. Cell 2016, 27, 2523–2527. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Munzberg, H. Novel aspects of brown adipose tissue biology. Endocrinol. Metab. Clin. 2013, 42, 89–107. [Google Scholar] [CrossRef] [Green Version]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, W.E.; Liu, X.; Bearden, C.M.; Harper, M.E.; Kozak, L.P. Effects of genetic background on thermoregulation and fatty acid-induced uncoupling of mitochondria in UCP1-deficient mice. J. Biol. Chem. 2001, 276, 12460–12465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townsend, K.L.; An, D.; Lynes, M.D.; Huang, T.L.; Zhang, H.; Goodyear, L.J.; Tseng, Y.H. Increased mitochondrial activity in BMP7-treated brown adipocytes, due to increased CPT1- and CD36-mediated fatty acid uptake. Antioxid. Redox Signal. 2013, 19, 243–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Q.; Zeng, F.; Liu, X.; Wang, Q.J.; Deng, F. Fatty acid oxidation and carnitine palmitoyltransferase I: Emerging therapeutic targets in cancer. Cell Death Dis. 2016, 7, e2226. [Google Scholar] [CrossRef]
- Seale, P.; Conroe, H.M.; Estall, J.; Kajimura, S.; Frontini, A.; Ishibashi, J.; Cohen, P.; Cinti, S.; Spiegelman, B.M. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Investig. 2011, 121, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scime, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Cohen, P.; Levy, J.D.; Zhang, Y.; Frontini, A.; Kolodin, D.P.; Svensson, K.J.; Lo, J.C.; Zeng, X.; Ye, L.; Khandekar, M.J.; et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014, 156, 304–316. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Hyun, C.K.; Kim, I.Y.; Frost, S.C. Soluble fibroin enhances insulin sensitivity and glucose metabolism in 3T3-L1 adipocytes. J. Nutr. 2004, 134, 3257–3263. [Google Scholar] [CrossRef] [Green Version]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.Y.; Lee, H.S.; Lee, H.J.; Kim, J.M.; Lee, K.W.; Suh, H.J. Feeding silk protein hydrolysates to C57BL/KsJ-db/db mice improves blood glucose and lipid profiles. Nutr. Res. 2010, 30, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Do, S.G.; Park, J.H.; Nam, H.; Kim, J.B.; Lee, J.Y.; Oh, Y.S.; Suh, J.G. Silk fibroin hydrolysate exerts an anti-diabetic effect by increasing pancreatic beta cell mass in C57BL/KsJ-db/db mice. J. Vet. Sci. 2012, 13, 339–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Lee, H.J.; Suh, H.J. Silk protein hydrolysate increases glucose uptake through up-regulation of GLUT 4 and reduces the expression of leptin in 3T3-L1 fibroblast. Nutr. Res. 2011, 31, 937–943. [Google Scholar] [CrossRef]
- Seo, C.W.; Um, I.C.; Rico, C.W.; Kang, M.Y. Antihyperlipidemic and body fat-lowering effects of silk proteins with different fibroin/sericin compositions in mice fed with high fat diet. J. Agric. Food Chem. 2011, 59, 4192–4197. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, H.; Yun, H.-Y.; Kim, B.; Lee, C.-H.; Suh, H.; Lim, K. Silk peptide intake increases fat oxidation at rest in exercised mice. J. Nutr. Sci. Vitaminol. 2013, 59, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, J.; Kim, B.; Lee, C.-H.; Lim, K.; Suh, H. Effects of different doses of silk peptide on energy metabolism during exercise in mice. J. Exerc. Nutr. Biochem. 2017, 21, 21. [Google Scholar] [CrossRef]
- Lee, K.; Seo, Y.-J.; Song, J.-H.; Lee, B.-Y. Ginsenoside Rg1 promotes browning by inducing UCP1 expression and mitochondrial activity in 3T3-L1 and subcutaneous white adipocytes. J. Ginseng Res. 2018, 43, 589–599. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.C. Enhancing energy and glucose metabolism by disrupting triglyceride synthesis: Lessons from mice lacking DGAT1. Nutr. Metab. 2006, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Calderon-Dominguez, M.; Mir, J.F.; Fucho, R.; Weber, M.; Serra, D.; Herrero, L. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte 2016, 5, 98–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Hurtado, E.; Lee, J.; Choi, J.; Wolfgang, M.J. Fatty acid oxidation is required for active and quiescent brown adipose tissue maintenance and thermogenic programing. Mol. Metab. 2018, 7, 45–56. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morak, M.; Schmidinger, H.; Riesenhuber, G.; Rechberger, G.N.; Kollroser, M.; Haemmerle, G.; Zechner, R.; Kronenberg, F.; Hermetter, A. Adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) deficiencies affect expression of lipolytic activities in mouse adipose tissues. Mol. Cell. Proteom. 2012, 11, 1777–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, P.; Spiegelman, B.M. Brown and beige fat: Molecular parts of a thermogenic machine. Diabetes 2015, 64, 2346–2351. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Zhu, M.; Rodgers, B.D.; Jiang, Q.; Dodson, M.V.; Du, M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int. J. Obes. 2015, 39, 967. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Liao, X.; Meng, F.; Wang, Y.; Sun, Z.; Guo, F.; Li, X.; Meng, M.; Li, Y.; Sun, C. Therapeutic role of ursolic acid on ameliorating hepatic steatosis and improving metabolic disorders in high-fat diet-induced non-alcoholic fatty liver disease rats. PLoS ONE 2014, 9, e86724. [Google Scholar] [CrossRef] [Green Version]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Alvarez-Crespo, M.; Csikasz, R.I.; Martínez-Sánchez, N.; Diéguez, C.; Cannon, B.; Nedergaard, J.; López, M. Essential role of UCP1 modulating the central effects of thyroid hormones on energy balance. Mol. Metab. 2016, 5, 271–282. [Google Scholar] [CrossRef]
- Lopez, M.; Varela, L.; Vazquez, M.J.; Rodriguez-Cuenca, S.; Gonzalez, C.R.; Velagapudi, V.R.; Morgan, D.A.; Schoenmakers, E.; Agassandian, K.; Lage, R.; et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat. Med. 2010, 16, 1001–1008. [Google Scholar] [CrossRef]
- Habinowski, S.A.; Witters, L.A. The effects of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2001, 286, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-activated protein kinase (AMPK) regulates energy metabolism through modulating thermogenesis in adipose tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, N.J.; Chang, S.H.; Li, D.Y.; Villanueva, C.J.; Park, K.W. Induction of thermogenic adipocytes: Molecular targets and thermogenic small molecules. Exp. Mol. Med. 2017, 49, e353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhang, H.; Li, B.; Meng, X.; Wang, J.; Zhang, Y.; Yao, S.; Ma, Q.; Jin, L.; Yang, J.; et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 2014, 5, 5493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Aita, S.; Kawai, Y.; Iwanaga, T.; Saito, M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am. J. Clin. Nutr. 2012, 95, 845–850. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, J.; Kim, B.; Lee, C.-H.; Lim, K.; Suh, H. Effects of silk peptides administration on fat utilization over a whole day in mice. J. Exerc. Nutr. Biochem. 2016, 20, 53. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Chon, J.-W.; Lee, K.-G.; Park, Y.-K.; Park, K.-H.; Yeo, J.-H. Anti-adipogenic Effect of Hydrolysate Silk Fibroin in 3T3-L1 Cells. Int. J. Ind. Entomol. 2010, 21, 169–174. [Google Scholar]
- Park, J.H.; Jung, H.; Nam, H.; Kim, J.-B.; Choe, N.-H.; Suh, J.-G. Silk fibroin hydrolysate ameliorates diabetic dyslipidemia in type 2 diabetic model mice. Anim. Cells Syst. 2013, 17, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Pan, M.H.; Hung, W.L.; Tung, Y.C.; Ho, C.T. From white to beige adipocytes: Therapeutic potential of dietary molecules against obesity and their molecular mechanisms. Food Funct. 2019, 10, 1263–1279. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Scherer, P.E. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol. Metab. 2012, 23, 435–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, C.W.; Ootsuka, Y.; Romanovsky, A.A. Body Temperature Measurements for Metabolic Phenotyping in Mice. Front. Physiol. 2017, 8, 520. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. Measuring energy metabolism in the mouse-theoretical, practical, and analytical considerations. Front. Physiol. 2013, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Rahman, O.; Kristóf, E.; Doan-Xuan, Q.-M.; Vida, A.; Nagy, L.; Horváth, A.; Simon, J.; Maros, T.; Szentkirályi, I.; Palotás, L. AMP-activated kinase (AMPK) activation by AICAR in human white adipocytes derived from pericardial white adipose tissue stem cells induces a partial beige-like phenotype. PLoS ONE 2016, 11, e0157644. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Jia, Y.; Fu, T.; Viswakarma, N.; Bai, L.; Rao, M.S.; Zhu, Y.; Borensztajn, J.; Reddy, J.K. Sustained activation of PPARα by endogenous ligands increases hepatic fatty acid oxidation and prevents obesity in ob/ob mice. FASEB J. 2012, 26, 628–638. [Google Scholar] [CrossRef] [Green Version]
- Schottl, T.; Kappler, L.; Braun, K.; Fromme, T.; Klingenspor, M. Limited mitochondrial capacity of visceral versus subcutaneous white adipocytes in male C57BL/6N mice. Endocrinology 2015, 156, 923–933. [Google Scholar] [CrossRef]
- Shabalina, I.G.; Petrovic, N.; de Jong, J.M.; Kalinovich, A.V.; Cannon, B.; Nedergaard, J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013, 5, 1196–1203. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.H.; Kim, K.J.; Kim, S.U.; Han, S.H.; Han, K.-H.; Cha, B.-S.; Chung, C.H.; Lee, B.-W. Obesity is more closely related with hepatic steatosis and fibrosis measured by transient elastography than metabolic health status. Metab. Clin. Exp. 2017, 66, 23–31. [Google Scholar] [CrossRef]
- Mottillo, E.P.; Desjardins, E.M.; Fritzen, A.M.; Zou, V.Z.; Crane, J.D.; Yabut, J.M.; Kiens, B.; Erion, D.M.; Lanba, A.; Granneman, J.G. FGF21 does not require adipocyte AMP-activated protein kinase (AMPK) or the phosphorylation of acetyl-CoA carboxylase (ACC) to mediate improvements in whole-body glucose homeostasis. Mol. Metab. 2017, 6, 471–481. [Google Scholar] [CrossRef]










| Nutrition Facts | Content (Dry Basis %) |
|---|---|
| Carbohydrate | 6.78 |
| Sugar | 0.94 |
| Fat | 0.01 |
| Protein | 86.80 |
| Sodium | 1.79 |
| Gene | Direction | Sequence (5′–3′) |
|---|---|---|
| 18s | Forward | GCAATTATTCCCCATGAACG |
| Reverse | GGCCTCACTAAACCATCCAA | |
| Acox1 | Forward | GCACCTTCGAGGGGGAGAACA |
| Reverse | GCGCGAACAAGGTCGACAGAA | |
| ATGL | Forward | TTGGTTCAGTAGGCCATTCC |
| Reverse | ACAGTGTCCCCATTCTCAGG | |
| Cyp4a10 | Forward | TTCAGAGCCTCCTGGGGGAT |
| Reverse | GGAGCAGTGTCAGGGCCACAA | |
| Cyp4a14 | Forward | ATGCCTGCCAGATTGCTCACG |
| Reverse | GGGTGGGTGGCCAGAGCATAG | |
| HSL | Forward | AGACACCAGCCAACGGATAC |
| Reverse | ATCACCCTCGAAGAAGAGCA | |
| NRF1 | Forward | TTGGAACAGCAGTGGCAAGA |
| Reverse | CTCACTTGCTGATGTATTTACTTCCAT | |
| PGC1α | Forward | ATGTGTCGCCTTCTTGCTCT |
| Reverse | ATCTACTGCCTGGGGACCTT | |
| PRDM16 | Forward | GATGGGAGATGCTGACGGAT |
| Reverse | TGATCTGACACATGGCGAGG | |
| TFAM | Forward | GTCGCATCCCCTCGTCTATC |
| Reverse | GCTGGAAAAACACTTCGGAATAC | |
| UCP1 | Forward | ACTGCCACACCTCCAGTCAT |
| Reverse | CTTTGCCTCACTCAGGATTG |
| Amino Acid | Content (Dry Basis %) |
|---|---|
| Glycine | 33.06 |
| Alanine | 28.09 |
| Serine | 11.09 |
| Valine | 2.67 |
| Tyrosine | 2.46 |
| Aspartic acid | 2.45 |
| Glutamic acid | 1.78 |
| Threonine | 1.22 |
| Cystein | 1.04 |
| Isoleucine | 0.76 |
| Proline | 0.74 |
| Luecine | 0.72 |
| Arginine | 0.50 |
| Phenylalanine | 0.44 |
| Lysine | 0.38 |
| Histidine | 0.38 |
| Methionine | 0.08 |
| Group | Blood Parameter (mg/dL) | |||
|---|---|---|---|---|
| CD | HFD | HFD | HFD | |
| Silk Peptide 0 * | Silk Peptide 0 * | Silk Peptide 50 * | Silk Peptide 200 * | |
| Triglycerides | 102.8 ± 5.3 b | 120.5 ± 6.0 a | 97.0 ± 20.0 b | 98.3 ± 7.5 b |
| Total cholesterol | 94.5 ± 7.9 b | 134.5 ± 5.4 a | 87.3 ± 18.0 b | 99.3 ± 19.0 b |
| LDL cholesterol | 11.5 ± 1.9 b | 14.8 ± 2.5 a | 9.0 ± 2.7 b | 9.3 ± 2.2 b |
| HDL cholesterol | 120.0 ± 8.2 a | 97.8 ± 6.4 b | 110.3 ± 13.2 a b | 116.3 ± 7.5 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Jin, H.; Chei, S.; Lee, J.-Y.; Oh, H.-J.; Lee, B.-Y. Dietary Silk Peptide Prevents High-Fat Diet-Induced Obesity and Promotes Adipose Browning by Activating AMP-Activated Protein Kinase in Mice. Nutrients 2020, 12, 201. https://doi.org/10.3390/nu12010201
Lee K, Jin H, Chei S, Lee J-Y, Oh H-J, Lee B-Y. Dietary Silk Peptide Prevents High-Fat Diet-Induced Obesity and Promotes Adipose Browning by Activating AMP-Activated Protein Kinase in Mice. Nutrients. 2020; 12(1):201. https://doi.org/10.3390/nu12010201
Chicago/Turabian StyleLee, Kippeum, Heegu Jin, Sungwoo Chei, Jeong-Yong Lee, Hyun-Ji Oh, and Boo-Yong Lee. 2020. "Dietary Silk Peptide Prevents High-Fat Diet-Induced Obesity and Promotes Adipose Browning by Activating AMP-Activated Protein Kinase in Mice" Nutrients 12, no. 1: 201. https://doi.org/10.3390/nu12010201