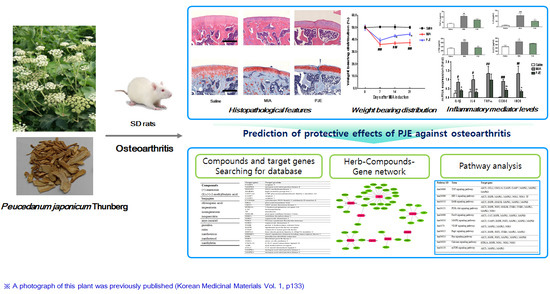
Protective Effects of Peucedanum japonicum Extract against Osteoarthritis in an Animal Model Using a Combined Systems Approach for Compound-Target Prediction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PJE
2.2. The MIA-Induced OA Rat Model
2.2.1. Animals
2.2.2. Induction of OA and Drug Treatment
2.2.3. Measurement of Hind Paw Weight-Bearing Distribution
2.2.4. Histopathological Analysis
2.2.5. Measurement of Serum Cytokine and Inflammatory Mediator Levels
2.2.6. Real-Time Quantitative RT-PCR Analysis
2.2.7. Statistical Analysis
2.3. Network Pharmacology Analysis
2.3.1. Screening of Active PJ Components
2.3.2. Pharmacokinetic Absorption, Distribution, Metabolism and Excretion (ADME) Evaluation
2.3.3. Identification of Associated Compounds and Target Genes
2.3.4. Network Construction and Analysis
3. Results
3.1. PJE Administration Restored the Hind Paw Weight-Bearing Distribution in MIA-Induced OA Rats
3.2. PJE Treatment Recovered the Histopathological Features of Joint Tissue in MIA-Induced OA Rats
3.3. PJE Administration Decreased Inflammatory Mediator Levels in Serum
3.4. PJE Treatment Inhibited mRNA Expression Levels of Inflammatory Mediators in MIA-Induced OA Rats
3.5. In Silico Network Analysis and Prediction of Target Genes and Pathways Related to OA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| PJ | Peucedanum japonicum |
| PJE | Peucedanum japonicum extract |
| MIA | Monosodium iodoacetate |
| CASP3 | Caspase-3 |
| CASP7 | Caspase-7 |
| CYP2D6 | Cytochrome P450 2D6 |
| TNF-α | Tumor necrosis factor |
| IL-6 | Interleukin-6 |
| LTB4 | Leukotriene B4 |
| 5-LOX | 5-lipoxygenase |
| OB | Oral bioavailability |
| DL | Drug-likeness |
| ADME | Absorption, distribution, metabolism, and excretion |
| TCMSP | Traditional Chinese Medicine Systems Pharmacology |
| STITCH | Search Tool for Interactions of Chemicals and Proteins |
| TTD | Therapeutic Targets Database |
| DAVID | Database for Annotation, Visualization, and Integrated Discovery |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| SIRT1 | Silent information regulator 1 |
References
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Huang, R. Topical treatment of degenerative knee osteoarthritis. Am. J. Med. Sci. 2018, 355, 6–12. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Regional Office for the Western Pacific. In Medicinal Plants in the Republic of Korea; Western Pacific Series No. 21; WHO Regional Office for the Western Pacific: Manila, Philippines, 1998; Volume 316, pp. 1976–1977. ISBN 9290611200. [Google Scholar]
- Kim, S.H.; Jong, H.S.; Yoon, M.H.; Oh, S.H.; Jung, K.T. Antinociceptive effect of intrathecal sec-O-glucosylhamaudol on the formalin-induced pain in rats. Korean J. Pain 2017, 30, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Nugara, R.N.; Inafuku, M.; Takara, K.; Iwasaki, H.; Oku, H. Pteryxin: A coumarin in Peucedanum japonicum thunb leaves exerts antiobesity activity through modulation of adipogenic gene network. Nutrition 2014, 30, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Taira, N.; Nugara, R.N.; Inafuku, M.; Takara, K.; Ogi, T.; Ichiba, T.; Iwasaki, H.; Okabe, T.; Oku, H. In vivo and in vitro anti-obesity activities of dihydropyranocoumarins derivatives from Peucedanum japonicum thunb. J. Funct. Foods 2017, 29, 19–28. [Google Scholar] [CrossRef]
- Kim, J.M.; Erkhembaatar, M.; Lee, G.S.; Lee, J.H.; Noh, E.M.; Lee, M.; Song, H.K.; Lee, C.H.; Kwon, K.B.; Kim, M.S.; et al. Peucedanum japonicum thunb. Ethanol extract suppresses RANKL-mediated osteoclastogenesis. Exp. Ther. Med. 2017, 14, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.M.; Lee, A.R.; Kim, H.S.; Lee, A.Y.; Gu, G.J.; Moon, B.C.; Kwon, B.I. Peucedanum japonicum extract attenuates allergic airway inflammation by inhibiting Th2 cell activation and production of pro-inflammatory mediators. J. Ethnopharmacol. 2018, 211, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.J.; Kim, J. Determination of the absolute configuration of khellactone esters from Peucedanum japonicum roots. J. Nat. Prod. 2017, 80, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.S.; Chang, C.T.; Sheen, W.S.; Teng, C.M.; Tsai, I.L.; Duh, C.Y.; Ko, F.N. Coumarins and antiplatelet aggregation constituents from formosan Peucedanum japonicum. Phytochemistry 1996, 41, 525–530. [Google Scholar] [CrossRef]
- Huong, D.T.; Choi, H.C.; Rho, T.C.; Lee, H.S.; Lee, M.K.; Kim, Y.H. Inhibitory activity of monoamine oxidase by coumarins from Peucedanum japonicum. Arch. Pharm. Res. 1999, 22, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Hisamoto, M.; Kikuzaki, H.; Ohigashi, H.; Nakatani, N. Antioxidant compounds from the leaves of Peucedanum japonicum thunb. J. Agric. Food Chem. 2003, 51, 5255–5261. [Google Scholar] [CrossRef] [PubMed]
- Hisamoto, M.; Kikuzaki, H.; Nakatani, N. Constituents of the leaves of Peucedanum japonicum thunb. and their biological activity. J. Agric. Food Chem. 2004, 52, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Choi, S.Z.; Lee, J.H.; Chung, S.H.; Park, S.H.; Kang, H.C.; Yang, E.Y.; Cho, H.J.; Lee, K.R. Antidiabetic coumarin and cyclitol compounds from Peucedanum japonicum. Arch. Pharm. Res. 2004, 27, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.B.; Li, Q.Y.; Chen, Q.L.; Su, S.B. Network pharmacology: A new approach for Chinese herbal medicine research. Evid.-Based Complement. Altern. Med. 2013, 2013, 621423. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; He, S.; Zhang, X.; Luo, S.; Zhang, B.; Duan, X.; Zhang, Z.; Wang, W.; Wang, Y.; Sun, Y. A network pharmacology approach to uncover the pharmacological mechanism of Xuanhusuo powder on osteoarthritis. Evid.-Based Complement. Altern. Med. 2016, 2016, 3246946. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Li, S.; Wang, N.; Tan, H.Y.; Cheung, F.; Feng, Y. A biomedical investigation of the hepatoprotective effect of Radix salviae miltiorrhizae and network pharmacology-based prediction of the active compounds and molecular targets. Int. J. Mol. Sci. 2017, 18, 620. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.L.; He, Y.M. Network pharmacology analysis of the anti-cancer pharmacological mechanisms of Ganoderma lucidum extract with experimental support using Hepa1-6-bearing C57 BL/6 mice. J. Ethnopharmacol. 2018, 210, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.M.; Kim, H.S.; Lee, A.Y.; Kim, S.H.; Kim, H.K. Anti-inflammatory and antiosteoarthritis effects of Saposhnikovia divaricata ethanol extract: In vitro and in vivo studies. Evid.-Based Complement. Altern. Med. 2016, 2016, 1984238. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.J.; Watkins, L.; Li, Z. Vasoactive intestinal peptide (VIP) is a modulator of joint pain in a rat model of osteoarthritis. Pain 2006, 123, 98–105. [Google Scholar] [CrossRef] [PubMed]
- PubMed. Available online: https://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 16 April 2018).
- Korean Traditional Knowledge Portal. Available online: http://www.koreantk.com/ktkp2014/ (accessed on 16 April 2018).
- ChEMBL. Available online: https://www.ebi.ac.uk/chembl/ (accessed on 16 April 2018).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 16 April 2018).
- Traditional Chinese Medicine Systems Pharmacology Database and Analysis. Available online: http://lsp.nwu.edu.cn/tcmsp.php (accessed on 16 April 2018).
- Wang, N.; Zheng, Y.; Gu, J.; Cai, Y.; Wang, S.; Zhang, F.; Chen, J.; Situ, H.; Lin, Y.; Wang, Z. Network-pharmacology-based validation of TAMS/CXCL-1 as key mediator of XIAOPI formula preventing breast cancer development and metastasis. Sci. Rep. 2017, 7, 14513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.C.; Agrawal, D.C.; Lee, M.R.; Lee, R.J.; Kuo, C.L.; Wu, C.R.; Tsay, H.S.; Chang, H.C. Influence of LED light spectra on in vitro somatic embryogenesis and LC-MS analysis of chlorogenic acid and rutin in Peucedanum japonicum thunb.: A medicinal herb. Bot. Stud. 2016, 57, 9. [Google Scholar] [CrossRef] [PubMed]
- Search Tool for Interactions of Chemicals and Proteins (STITCH). Available online: http://stitch.embl.de/ (accessed on 20 April 2018).
- von Mering, C.; Jensen, L.J.; Snel, B.; Hooper, S.D.; Krupp, M.; Foglierini, M.; Jouffre, N.; Huynen, M.A.; Bork, P. String: Known and predicted protein-protein associations, integrated and transferred across organisms. Nucleic Acids Res. 2005, 33, D433–D437. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Xia, T.; Wang, L.; Zhao, Q.; Tian, J. Investigation of candidate genes for osteoarthritis based on gene expression profiles. Acta Orthop. Traumatol. Turc. 2016, 50, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Summer, G.; Kelder, T.; Radonjic, M.; van Bilsen, M.; Wopereis, S.; Heymans, S. The network library: A framework to rapidly integrate network biology resources. Bioinformatics 2016, 32, i473–i478. [Google Scholar] [CrossRef] [PubMed]
- Therapeutic Targets Database (TTD). Available online: http://bidd.nus.edu.sg/BIDD-Databases/TTD/TTD.asp (accessed on 20 April 2018).
- Cytoscape. Available online: http://www.cytoscape.org/ (accessed on 24 April 2018).
- Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.L.; Ideker, T. Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics 2011, 27, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Database for Annotation, Visualization, and Integrated Discovery (DAVID). Available online: https://david.ncifcrf.gov/ (accessed on 24 April 2018).
- Kyoto Encyclopedia of Genes and Genomes (KEGG). Available online: http://www.genome.jp/kegg/ (accessed on 24 April 2018).
- Bove, S.E.; Calcaterra, S.L.; Brooker, R.M.; Huber, C.M.; Guzman, R.E.; Juneau, P.L.; Schrier, D.J.; Kilgore, K.S. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr. Cartil. 2003, 11, 821–830. [Google Scholar] [CrossRef]
- Paquet, J.; Goebel, J.C.; Delaunay, C.; Pinzano, A.; Grossin, L.; Cournil-Henrionnet, C.; Gillet, P.; Netter, P.; Jouzeau, J.Y.; Moulin, D. Cytokines profiling by multiplex analysis in experimental arthritis: Which pathophysiological relevance for articular versus systemic mediators? Arth. Res. Ther. 2012, 14, R60. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Med. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Daghestani, H.N.; Kraus, V.B. Inflammatory biomarkers in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Blanco, F.J.; Cornelisson, M.; Lotz, M. Regulation of cyclooxygenase-2 expression in normal human articular chondrocytes. J. Immunol. 1995, 155, 796–801. [Google Scholar] [PubMed]
- Martel-Pelletier, J.; Lajeunesse, D.; Reboul, P.; Pelletier, J.P. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann. Rheum. Dis. 2003, 62, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Na, J.Y.; Song, K.; Kim, S.; Kwon, J. Rutin protects rat articular chondrocytes against oxidative stress induced by hydrogen peroxide through SIRT1 activation. Biochem. Biophys. Res. Commun. 2016, 473, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.P.; Tang, J.L.; Bao, J.P.; Hu, P.F.; Shi, Z.L.; Wu, L.D. Anti-arthritic effects of chlorogenic acid in interleukin-1beta-induced rabbit chondrocytes and a rabbit osteoarthritis model. Int. Immunopharmacol. 2011, 11, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Bai, Y.; Liu, C.; Dou, C.; Li, J.; Xiang, J.; Zhao, C.; Xie, Z.; Xiang, Q.; Dong, S. Hypertrophic differentiation of mesenchymal stem cells is suppressed by xanthotoxin via the p38MAPK/HDAC4 pathway. Mol. Med. Rep. 2017, 16, 2740–2746. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.P.; Martel-Pelletier, J.; Abramson, S.B. Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets. Arthritis Rheumatol. 2001, 44, 1237–1247. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.S.; Kim, H.A. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef] [PubMed]
- Dean, L. Tramadol therapy and cyp2d6 genotype. In Medical Genetics Summaries; Pratt, V., McLeod, H., Dean, L., Malheiro, A., Rubinstein, W., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Maksymowych, W.P.; Russell, A.S.; Chiu, P.; Yan, A.; Jones, N.; Clare, T.; Lambert, R.G. Targeting tumour necrosis factor alleviates signs and symptoms of inflammatory osteoarthritis of the knee. Arth. Res. Ther. 2012, 14, R206. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Torres, J.; Martinez-Nava, G.A.; Gutierrez-Ruiz, M.C.; Gomez-Quiroz, L.E.; Gutierrez, M. Role of HIF-1alpha signaling pathway in osteoarthritis: A systematic review. Rev. Bras. Reumatol. 2017, 57, 162–173. [Google Scholar]
- Fu, D.; Shang, X.; Ni, Z.; Shi, G. Shikonin inhibits inflammation and chondrocyte apoptosis by regulation of the PI3K/Akt signaling pathway in a rat model of osteoarthritis. Exp. Ther. Med. 2016, 12, 2735–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeser, R.F.; Erickson, E.A.; Long, D.L. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Curr. Opin. Rheumatol. 2008, 20, 581–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, M.; Yudoh, K.; Masuko, K. The potential role of vascular endothelial growth factor (VEGF) in cartilage: How the angiogenic factor could be involved in the pathogenesis of osteoarthritis? Osteoarthr. Cartil. 2008, 16, 279–286. [Google Scholar] [CrossRef] [PubMed]





| Gene | Primer Sequence | Accession No. | |
|---|---|---|---|
| IL-1β | Forward Reverse | 5′-CCCTGCAGCTGGAGAGTGTGG-3′ 5′-TGTGCTCTGCTTGAGAGGTGCT-3′ | NM_031512.2 |
| IL-6 | Forward Reverse | 5′-TTCCTACCCCAACTTCCAATG-3′ 5′-ATGAGTTGGATGGTCTTGGTC-3′ | NM_012589.1 |
| TNF-α | Forward Reverse | 5′-GACCCTCACACTCAGATCATCTTCT-3′ 5′-TGCTACGACGTGGGCTACG-3′ | NM_012675.3 |
| COX-2 | Forward Reverse | 5′-TGGTGCCGGGTCTGATGATG-3′ 5′-GCAATGCGGTTCTGATACTG-3′ | S67722.1 |
| iNOS | Forward Reverse | 5′-CTTTACGCCACTAACAGTGGCA-3′ 5′-AGTCATGCTTCCCATCGCTC-3′ | NM_012611.3 |
| GAPDH | Probe | Applied Biosystems Rat GAPD (GAPDH) Endogenous Control (VIC/MGB Probe, 4352338E) |
| Pathway Classification | Pathway ID | Term | Target Gene |
|---|---|---|---|
| Signal transduction | hsa04668 | TNF signaling pathway | AKT1, CCL2, CXCL10, CASP3, CASP7, MAPK1, MAPK3, MAPK8 |
| Signal transduction | hsa04066 | HIF-1 signaling pathway | AKT1, EGFR, MAPK1, MAPK3, NOS2, NOS3, TF |
| Signal transduction | has04310 | ErbB signaling pathway | AKT1, EGFR, GSK3B, MAPK1, MAPK3, MAPK8 |
| Signal transduction | has04151 | PI3K-Akt signaling pathway | AKT1, EGFR, FGF2, GSK3B, ITGB3, ITGB5, MAPK1, MAPK3, NOS3 |
| Signal transduction | hsa04068 | FoxO signaling pathway | AKT1, CAT, EGFR, MAPK1, MAPK3, MAPK8 |
| Signal transduction | hsa04010 | MAPK signaling pathway | AKT1, CASP3, EGFR, FGF2, MAPK1, MAPK3, MAPK8 |
| Signal transduction | hsa4370 | VEGF signaling pathway | AKT1, MAPK1, MAPK3, NOS3 |
| Signal transduction | hsa04015 | Rap1 signaling pathway | AKT1, EGFR, FGF2, ITGB3, MAPK1, MAPK3 |
| Signal transduction | hsa04014 | Ras signaling pathway | AKT1, EGFR, FGF2, MAPK1, MAPK3, MAPK8 |
| Signal transduction | hsa04020 | Calcium signaling pathway | HTR2A, EGFR, NOS1, NOS2, NOS3 |
| Signal transduction | hsa04150 | mTOR signaling pathway | AKT1, MAPK1, MAPK3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, J.M.; Lee, A.Y.; Kim, J.S.; Choi, G.; Kim, S.-H. Protective Effects of Peucedanum japonicum Extract against Osteoarthritis in an Animal Model Using a Combined Systems Approach for Compound-Target Prediction. Nutrients 2018, 10, 754. https://doi.org/10.3390/nu10060754
Chun JM, Lee AY, Kim JS, Choi G, Kim S-H. Protective Effects of Peucedanum japonicum Extract against Osteoarthritis in an Animal Model Using a Combined Systems Approach for Compound-Target Prediction. Nutrients. 2018; 10(6):754. https://doi.org/10.3390/nu10060754
Chicago/Turabian StyleChun, Jin Mi, A Yeong Lee, Joong Sun Kim, Goya Choi, and Seung-Hyung Kim. 2018. "Protective Effects of Peucedanum japonicum Extract against Osteoarthritis in an Animal Model Using a Combined Systems Approach for Compound-Target Prediction" Nutrients 10, no. 6: 754. https://doi.org/10.3390/nu10060754