The Preparation and Properties of Multilayer Cu-MTa2O5 Composite Coatings on Ti6Al4V for Biomedical Applications
Abstract
:1. Introduction
2. Material and Experimental Methods
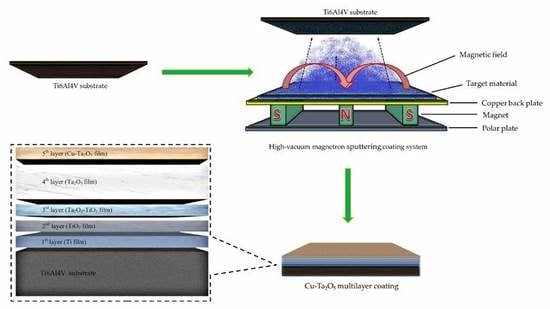
2.1. Coatings Deposition
2.2. Coatings Characterization
2.3. Scratch Test
2.4. Contact Angle Measurements
2.5. Electrochemical Experiments
2.6. Antibacterial Tests
2.7. Statistical Analysis
3. Results and Discussion
3.1. Microstructural Characterization
3.2. Adhesion Strength
3.3. Wettability
3.4. Corrosion Behavior
3.5. Antibacterial Property
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rahmati, B.; Zalnezhad, E.; Sarhan, A.A.D.; Kamiab, Z.; Tabrizi, B.N.; Abas, W.A.B.W. Enhancing the adhesion strength of tantalum oxide ceramic thin film coating on biomedical Ti–6Al–4V alloy by thermal surface treatment. Ceram. Int. 2015, 41, 13055–13063. [Google Scholar] [CrossRef]
- Markhoff, J.; Krogull, M.; Schulze, C.; Rotsch, C.; Hunger, S.; Bader, R. Biocompatibility and Inflammatory Potential of Titanium Alloys Cultivated with Human Osteoblasts, Fibroblasts and Macrophages. Materials 2017, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Xu, J.; Lu, X.; Hu, D.; Tao, H.; Munroe, P.; Xie, Z.-H. Corrosion and wear behaviours of a reactive-sputter-deposited Ta2O5 nanoceramic coating. Appl. Surf. Sci. 2016, 368, 177–190. [Google Scholar] [CrossRef]
- Ding, Z.; He, Q.; Ding, Z.; Liao, C.; Chen, D.; Ou, L. Fabrication and Performance of ZnO Doped Tantalum Oxide Multilayer Composite Coatings on Ti6Al4V for Orthopedic Application. Nanomaterials 2019, 9, 685. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Mumjitha, M.S. Fabrication of biopolymers reinforced TNT/HA coatings on Ti: Evaluation of its Corrosion resistance and Biocompatibility. Electrochim. Acta 2015, 153, 1–11. [Google Scholar] [CrossRef]
- Ananth, K.P.; Suganya, S.; Mangalaraj, D.; Ferreira, J.M.; Balamurugan, A. Electrophoretic bilayer deposition of zirconia and reinforced bioglass system on Ti6Al4V for implant applications: An in vitro investigation. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4160–4166. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards Improvements for Penetrating the Blood-Brain Barrier-Recent Progress from a Material and Pharmaceutical Perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef]
- Au, A.; Ha, J.; Hernandez, M.; Polotsky, A.; Hungerford, D.S.; Frondoza, C.G. Nickel and vanadium metal ions induce apoptosis of T-lymphocyte Jurkat cells. J. Biomed. Mater. Res. Part A 2006, 79, 512–521. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Meng, F.; Chu, P.K. Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials 2011, 32, 693–705. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Ghosh, R.; Swart, O.; Westgate, S.; Miller, B.L.; Yates, M.Z. Antibacterial Copper-Hydroxyapatite Composite Coatings via Electrochemical Synthesis. Langmuir ACS J. Surf. Colloids 2019, 35, 5957–5966. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Jones, I.P.; Landini, G.; Mei, J.; Tse, Y.Y.; Li, Y.X.; Ke, L.; Huang, Y.; Liu, L.I.; Wang, C.; et al. Backscattered electron imaging and electron backscattered diffraction in the study of bacterial attachment to titanium alloy structure. J. Microsc. 2018, 270, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Lin, G.; Gong, S.; Liu, X.; Sun, G.; Wu, H. Mechanical and corrosive characteristics of Ta/TaN multilayer coatings. Vacuum 2013, 89, 244–248. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. A promising sensing platform toward dopamine using MnO2 nanowires/electro-reduced graphene oxide composites. Electrochim. Acta 2019, 296, 683–692. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Manganese dioxide Nanorods/electrochemically reduced graphene oxide nanocomposites modified electrodes for cost-effective and ultrasensitive detection of Amaranth. Colloids Surf. B Biointerfaces 2018, 172, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, P.; Tian, Y.; Magesa, F.; Liu, J.; Li, G.; He, Q. Construction of effective electrochemical sensor for the determination of quinoline yellow based on different morphologies of manganese dioxide functionalized graphene. J. Food Compos. Anal. 2019, 84, 103280. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. Fabrication of Amine-Modified Magnetite-Electrochemically Reduced Graphene Oxide Nanocomposite Modified Glassy Carbon Electrode for Sensitive Dopamine Determination. Nanomaterials 2018, 8, 194. [Google Scholar] [CrossRef]
- He, Q.; Wu, Y.; Tian, Y.; Li, G.; Liu, J.; Deng, P.; Chen, D. Facile electrochemical sensor for nanomolar rutin detection based on magnetite nanoparticles and reduced graphene oxide decorated electrode. Nanomaterials 2019, 9, 115. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Ding, Z.; Li, G.; Liu, J.; Zuberi, Z.; He, Q. Rapid recognition and determination of tryptophan by carbon nanotubes and molecularly imprinted polymer-modified glassy carbon electrode. Bioelectrochemistry 2019, 130, 107393. [Google Scholar] [CrossRef]
- Hsieh, J.H.; Yeh, T.H.; Hung, S.Y.; Chang, S.Y.; Wu, W.; Li, C. Antibacterial and tribological properties of TaN–Cu, TaN–Ag, and TaN–(Ag,Cu) nanocomposite thin films. Mater. Res. Bull. 2012, 47, 2999–3003. [Google Scholar] [CrossRef]
- Hee, A.C.; Cao, H.; Zhao, Y.; Jamali, S.S.; Bendavid, A.; Martin, P.J. Cytocompatible tantalum films on Ti6Al4V substrate by filtered cathodic vacuum arc deposition. Bioelectrochemistry 2018, 122, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Krishna, D.S.R.; Sun, Y.; Chen, Z. Magnetron sputtered TiO2 films on a stainless steel substrate: Selective rutile phase formation and its tribological and anti-corrosion performance. Thin Solid Films 2011, 519, 4860–4864. [Google Scholar] [CrossRef]
- Lopes, N.I.d.A.; de Arruda Santos, L.; Lopes Buono, V.T. Mechanical Properties of Nanoceramic Zirconia Coatings on NiTi Orthodontic Wires. Adv. Sci. Technol. 2016, 97, 147–152. [Google Scholar] [CrossRef]
- Hernández Rodríguez, E.N.; Íñiguez Contreras, C.M.; Márquez Herrera, A.; Zapata Torres, M.G.; Balvantín Gracía, A.; Diosdado De la Peña, J.A.; Mis Fernández, R.; Peña Chapa, J.L. Development of MgOx–TiOx coatings for modulation of Mg corrosion resistance. Metall. Res. Technol. 2018, 115, 205. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Rameshbabu, N.; Bose, A.C.; Muthupandi, V.; Subramanian, S.; MubarakAli, D.; Thajuddin, N. Fabrication of corrosion resistant, bioactive and antibacterial silver substituted hydroxyapatite/titania composite coating on Cp Ti. Ceram. Int. 2012, 38, 731–740. [Google Scholar] [CrossRef]
- Duraipandy, N.; Syamala, K.M.; Rajendran, N. Antibacterial effects, biocompatibility and electrochemical behavior of zinc incorporated niobium oxide coating on 316L SS for biomedical applications. Appl. Surf. Sci. 2018, 427, 1166–1181. [Google Scholar]
- Xu, J.; Hu, W.; Xie, Z.-H.; Munroe, P. Reactive-sputter-deposited β-Ta2O5 and TaON nanoceramic coatings on Ti–6Al–4V alloy against wear and corrosion damage. Surface and Coatings Technology 2016, 296, 171–184. [Google Scholar] [CrossRef]
- Xu, J.; Bao, X.k.; Fu, T.; Lyu, Y.; Munroe, P.; Xie, Z.-H. In vitro biocompatibility of a nanocrystalline β-Ta2O5 coating for orthopaedic implants. Ceram. Int. 2018, 44, 4660–4675. [Google Scholar] [CrossRef]
- Rahmati, B.; Sarhan, A.A.D.; Zalnezhad, E.; Kamiab, Z.; Dabbagh, A.; Choudhury, D.; Abas, W.A.B.W. Development of tantalum oxide (Ta-O) thin film coating on biomedical Ti-6Al-4V alloy to enhance mechanical properties and biocompatibility. Ceram. Int. 2016, 42, 466–480. [Google Scholar] [CrossRef]
- Antoniac, I.V. Handbook of Bioceramics and Biocomposites; Springer: Berlin, Germany, 2016. [Google Scholar]
- Hogmark, S.; Jacobson, S.; Larsson, M. Design and evaluation of tribological coatings. Wear 2000, 246, 20–33. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, M.; Ding, C. Bond strength of plasma-sprayed hydroxyapatite/Ti composite coatings. Biomaterials 2000, 21, 841–849. [Google Scholar] [CrossRef]
- Berezhnaya, A.Y.; Mittova, V.O.; Kukueva, E.V.; Mittova, I.Y. Effect of high-temperature annealing on solid-state reactions in hydroxyapatite/TiO2films on titanium substrates. Inorg. Mater. 2010, 46, 971–977. [Google Scholar] [CrossRef]
- Chou, B.Y.; Chang, E. Influence of deposition temperature on mechanical properties of plasma-sprayed hydroxyapatite coating on titanium alloy with ZrO2 intermediate layer. J. Therm. Spray Technol. 2003, 12, 199–207. [Google Scholar] [CrossRef]
- Azem, F.A.; Birlik, I.; Braic, V.; Toparli, M.; Celik, E.; Parau, A.; Kiss, A.; Titorencu, I.; Vladescu, A. Effect of SiC interlayer between Ti6Al4V alloy and hydroxyapatite films. Proc. Inst. Mech. Eng. H 2015, 229, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yang, Y.; Zhou, M.; Chen, Z.; Chen, K. Effect of transition layer on the performance of hydroxyapatite/titanium nitride coating developed on Ti-6Al-4V alloy by magnetron sputtering. Ceram. Int. 2018, 45, 4863–4869. [Google Scholar] [CrossRef]
- Quirama, A.; Echavarría, A.M.; Meza, J.; Osorio, J.; Bejarano, G. Improvement of the mechanical behavior of the calcium phosphate coatings deposited onto Ti6Al4V alloy using an intermediate TiN/TiO2 bilayer. Vacuum 2017, 146, 22–30. [Google Scholar] [CrossRef]
- Bai, W.Q.; Li, L.L.; Li, R.L.; Gu, C.D.; Wang, X.L.; Jin, G.; Liu, D.G.; Tu, J.P. Deposition and characterization of a ZrN/Zr/a-C multilayer: Implication on bio-tribological and corrosion behaviors. Surf. Coat. Technol. 2017, 324, 509–517. [Google Scholar] [CrossRef]
- Major, L.; Lackner, J.; Major, B. Bio-tribological TiN/Ti/aC: H multilayer coatings development with a built-in mechanism of controlled wear. RSC Adv. 2014, 4, 21108–21114. [Google Scholar] [CrossRef]
- Zhang, E.; Li, F.; Wang, H.; Liu, J.; Wang, C.; Li, M.; Yang, K. A new antibacterial titanium-copper sintered alloy: Preparation and antibacterial property. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4280–4287. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-reduced graphene nanocomposite modified electrodes towards ultrasensitive dopamine detection. Sensors 2018, 18, 199. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Wang, X.; Wang, Y.; Hang, R.; Huang, X.; Tang, B.; Chu, P.K. Effects of copper nanoparticles in porous TiO2 coatings on bacterial resistance and cytocompatibility of osteoblasts and endothelial cells. Mater. Sci. Eng. C 2018, 82, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Ewald, A.; Kappel, C.; Vorndran, E.; Moseke, C.; Gelinsky, M.; Gbureck, U. The effect of Cu(II)-loaded brushite scaffolds on growth and activity of osteoblastic cells. J. Biomed. Mater. Res. Part A 2012, 100, 2392–2400. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, Y.; Huddleston, S.; Li, P.; Xiao, B.; Farha, O.K.; Ameer, G.A. Copper Metal-Organic Framework Nanoparticles Stabilized with Folic Acid Improve Wound Healing in Diabetes. ACS Nano 2018, 12, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Lin, C.; Deng, Y.; Yuan, Z.; Shen, X.; Chen, M.; He, Y.; Peng, Z.; Hu, Y.; Cai, K. Copper-nanoparticle-embedded hydrogel for killing bacteria and promoting wound healing with photothermal therapy. J. Mater. Chem. B 2019, 7, 2534–2548. [Google Scholar] [CrossRef]
- He, X.; Zhang, G.; Wang, X.; Hang, R.; Huang, X.; Qin, L.; Tang, B.; Zhang, X. Biocompatibility, corrosion resistance and antibacterial activity of TiO2/CuO coating on titanium. Ceram. Int. 2017, 43, 16185–16195. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Yan, T.; Han, Y. Fibroblast responses and antibacterial activity of Cu and Zn co-doped TiO2 for percutaneous implants. Appl. Surf. Sci. 2018, 434, 633–642. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Huang, C.-F.; Ou, K.-L.; Peng, P.-W. Mechanical properties and antibacterial activity of copper doped diamond-like carbon films. Surf. Coat. Technol. 2011, 206, 1037–1040. [Google Scholar] [CrossRef]
- Rosenbaum, J.; Versace, D.L.; Abbad-Andallousi, S.; Pires, R.; Azevedo, C.; Cenedese, P.; Dubot, P. Antibacterial properties of nanostructured Cu-TiO2 surfaces for dental implants. Biomater. Sci. 2017, 5, 455–462. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Geng, Z.; Yin, Y.; Hang, R.; Huang, X.; Yao, X.; Tang, B. Preparation, antibacterial effects and corrosion resistant of porous Cu–TiO2 coatings. Appl. Surf. Sci. 2014, 308, 43–49. [Google Scholar] [CrossRef]
- Hong, I.T.; Koo, C.H. Antibacterial properties, corrosion resistance and mechanical properties of Cu-modified SUS 304 stainless steel. Mater. Sci. Eng. A 2005, 393, 213–222. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R.; Magid, H.A.; Goh, B.T.; Yoon, G.H. Ti/TiN/HA coating on Ti–6Al–4V for biomedical applications. Ceram. Int. 2015, 41, 14447–14457. [Google Scholar] [CrossRef]
- Michael, T.; Urquhart, A.J.; Mischa, Z.; Davies, M.C.; Alexander, M.R. Picoliter water contact angle measurement on polymers. Langmuir ACS J. Surf. Colloids 2007, 23, 6875. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Tang, Y.; Zeng, L.; Zhao, Y.; Ma, Z.; Sun, Z.; Xiang, L.; Ren, L.; Yang, K. In vitro and in vivo studies of anti-bacterial copper-bearing titanium alloy for dental application. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2018, 34, 1112–1126. [Google Scholar] [CrossRef]
- Barth, E.; Myrvik, Q.M.; Wagner, W.; Gristina, A.G. In vitro and in vivo comparative colonization of Staphylococcus aureus and Staphylococcus epidermidis on orthopaedic implant materials. Biomaterials 1989, 10, 325–328. [Google Scholar] [CrossRef]
- Balashabadi, P.; Larijani, M.; Jafari-Khamse, E.; Seyedi, H. The role of Cu content on the structural properties and hardness of TiN–Cu nanocomposite film. J. Alloy. Compd. 2017, 728, 863–871. [Google Scholar] [CrossRef]
- Cheng, Y.; Cao, L.; He, G.; Yao, G.; Song, X.; Sun, Z. Preparation, microstructure and photoelectrical properties of Tantalum-doped zinc oxide transparent conducting films. J. Alloy. Compd. 2014, 608, 85–89. [Google Scholar] [CrossRef]
- Wu, S.-J.J.; Houng, B.; Huang, B.-S. Effect of growth and annealing temperatures on crystallization of tantalum pentoxide thin film prepared by RF magnetron sputtering method. J. Alloy. Compd. 2009, 475, 488–493. [Google Scholar] [CrossRef]
- Haque, S.M.; Sagdeo, P.R.; Shinde, D.D.; Misal, J.S.; Jha, S.N.; Bhattacharyya, D.; Sahoo, N.K. Extended x-ray absorption fine structure measurements on asymmetric bipolar pulse direct current magnetron sputtered Ta(2)O(5) thin films. Appl. Opt. 2015, 54, 6744–6751. [Google Scholar] [CrossRef]
- Chen, H.; Ding, J.; Shi, F.; Li, Y.; Guo, W. Optical properties of Ti-doped ZnO films synthesized via magnetron sputtering. J. Alloy. Compd. 2012, 534, 59–63. [Google Scholar] [CrossRef]
- Bright, T.J.; Watjen, J.I.; Zhang, Z.M.; Muratore, C.; Voevodin, A.A.; Koukis, D.I.; Tanner, D.B.; Arenas, D.J. Infrared optical properties of amorphous and nanocrystalline Ta2O5 thin films. J. Appl. Phys. 2013, 114, 083515. [Google Scholar] [CrossRef]
- Donkov, N.; Mateev, E.; Safonov, V.; Zykova, A.; Yakovin, S.; Kolesnikov, D.; Sudzhanskaya, I.; Goncharov, I.; Georgieva, V. Comparative analysis of electrophysical properties of ceramic tantalum pentoxide coatings, deposited by electron beam evaporation and magnetron sputtering methods. J. Phys. Conf. Ser. 2014, 558, 012036. [Google Scholar] [CrossRef]
- Cano, E.; Torres, C.L.; Bastidas, J.M. An XPS study of copper corrosion originated by formic acid vapour at 40% and 80% relative humidity. Mater. Corros. 2015, 52, 667–676. [Google Scholar] [CrossRef]
- Laibinis, P.E.; Whitesides, G.M. Self- Assembled Monolayers of N-Alkanethiolates on Copper Are Barrier Films That Protect the Metal Against Oxidation by Air. J. Am. Chem. Soc. 1992, 114, 9022–9028. [Google Scholar] [CrossRef]
- Huang, H.-L.; Chang, Y.-Y.; Chen, H.-J.; Chou, Y.-K.; Lai, C.-H.; Chen, M.Y.C. Antibacterial properties and cytocompatibility of tantalum oxide coatings with different silver content. J. Vac. Sci. Technol. A Vac. Surf. Films 2014, 32, 02B117. [Google Scholar] [CrossRef]
- Kumar, A.M.; Khan, A.; Suleiman, R.; Qamar, M.; Saravanan, S.; Dafalla, H. Bifunctional CuO/TiO2 nanocomposite as nanofiller for improved corrosion resistance and antibacterial protection. Prog. Org. Coat. 2018, 114, 9–18. [Google Scholar] [CrossRef]
- Alotaibi, A.M.; Sathasivam, S.; Nair, S.P.; Parkin, I.P. Antibacterial properties of Cu-ZrO2 thin films prepared via aerosol assisted chemical vapour deposition. J. Mater. Chem. B 2016, 4, 666–671. [Google Scholar] [CrossRef]
- Quiros, J.; Borges, J.P.; Boltes, K.; Rodea-Palomares, I.; Rosal, R. Antimicrobial electrospun silver-, copper- and zinc-doped polyvinylpyrrolidone nanofibers. J. Hazard. Mater. 2015, 299, 298–305. [Google Scholar] [CrossRef]
- Tan, A.W.-Y.; Sun, W.; Bhowmik, A.; Lek, J.Y.; Marinescu, I.; Li, F.; Khun, N.W.; Dong, Z.; Liu, E. Effect of coating thickness on microstructure, mechanical properties and fracture behaviour of cold sprayed Ti6Al4V coatings on Ti6Al4V substrates. Surf. Coat. Technol. 2018, 349, 303–317. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Gao, L.; Liu, E.; Yu, F.; Shu, X.; Wang, H. Microstructure, corrosion and tribological and antibacterial properties of Ti-Cu coated stainless steel. J. Mech. Behav. Biomed. Mater. 2015, 50, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Dave, P.Y.; Patel, K.H.; Chauhan, K.V.; Chawla, A.K.; Rawal, S.K. Examination of Zinc Oxide Films Prepared by Magnetron Sputtering. Procedia Technol. 2016, 23, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.G.; Kaneko, J. Hydrophobicity and corrosion resistance of steels coated with PFDS film. Surf. Eng. 2013, 29, 255–263. [Google Scholar] [CrossRef]
- Rahmati, B.; Sarhan, A.A.D.; Basirun, W.J.; Abas, W.A.B.W. Ceramic tantalum oxide thin film coating to enhance the corrosion and wear characteristics of Ti6Al4V alloy. J. Alloy. Compd. 2016, 676, 369–376. [Google Scholar] [CrossRef]
- El-Rabiee, M.M.; Helal, N.H.; El-Hafez, G.M.A.; Badawy, W.A. Corrosion control of vanadium in aqueous solutions by amino acids. J. Alloy. Compd. 2008, 459, 466–471. [Google Scholar] [CrossRef]
- Prasad, S.; Ehrensberger, M.; Gibson, M.P.; Kim, H.; Monaco, E.A. Biomaterial properties of titanium in dentistry. J. Oral Biosci. 2015, 57, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Sakairi, M.; Jha, H. Influence of desiccation procedures on the surface wettability and corrosion resistance of porous aluminium anodic oxide films. Corros. Sci. 2012, 55, 332–338. [Google Scholar] [CrossRef]
- Black, J. Biological performance of tantalum. Clin. Mater. 1994, 16, 167. [Google Scholar] [CrossRef]
- Raghupathy, Y.; Kamboj, A.; Rekha, M.Y.; Rao, N.P.N.; Srivastava, C. Copper-graphene oxide composite coatings for corrosion protection of mild steel in 3.5% NaCl. Thin Solid Films 2017, 636, 107–115. [Google Scholar] [CrossRef]
- Patel, M.N.; Dosi, P.A.; Bhatt, B.S. Antibacterial, DNA interaction and superoxide dismutase activity of drug based copper(II) coordination compounds. Polyhedron 2010, 29, 3238–3245. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, B.; Liang, Y.; Zhang, J.; Yan, Y.; Chen, X.; Wu, Z.; Liu, H.; Wen, S.; Tan, S.; et al. Study on the antibacterial mechanism of copper ion- and neodymium ion-modified alpha-zirconium phosphate with better antibacterial activity and lower cytotoxicity. Colloids Surf. B Biointerfaces 2015, 132, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, Z.; Zhu, Y.; Xia, H.; Yao, M.; Chu, X.; Wang, X.; Yang, K.; Yang, M.; Zhang, Y.; et al. Toward a Molecular Understanding of the Antibacterial Mechanism of Copper-Bearing Titanium Alloys against Staphylococcus aureus. Adv. Healthc. Mater. 2016, 5, 557–566. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Huang, H.-L.; Chen, H.-J.; Lai, C.-H.; Wen, C.-Y. Antibacterial properties and cytocompatibility of tantalum oxide coatings. Surf. Coat. Technol. 2014, 259, 193–198. [Google Scholar] [CrossRef]
- Meidanchi, A.; Jafari, A. Synthesis and characterization of high purity Ta2O5 nanoparticles by laser ablation and its antibacterial properties. Opt. Laser Technol. 2019, 111, 89–94. [Google Scholar] [CrossRef]














| Composition | Al | V | Fe | O | C | N | H | Ti |
|---|---|---|---|---|---|---|---|---|
| wt % | 6.8 | 4.5 | 0.3 | 0.2 | 0.1 | 0.05 | 0.015 | Balance |
| Coating Code | Layer Number | Coating Material | Sputtering Mode | Sputtering Power (W) | Deposition Time (min) | Gas Flow (sccm) | ||
|---|---|---|---|---|---|---|---|---|
| Ar | O2 | |||||||
| Cu-MTa2O5 | 1st layer | Ti | DC sputtering | 200 | 8 | 20 | / | |
| 2nd layer | TiO2 | DC reactive sputtering | 200 | 8 | 16 | 4 | ||
| 3rd layer | Ta2O5-TiO2 | Ta2O5 | RF sputtering | 200 | 8 | 20 | 5 | |
| TiO2 | DC reactive sputtering | 200 | ||||||
| 4th layer | Ta2O5 | RF sputtering | 200 | 105 | 20 | / | ||
| 5th layer | Cu-Ta2O5 | Cu | DC sputtering | 80 | 15 | 20 | / | |
| Ta2O5 | RF sputtering | 200 | ||||||
| Ta2O5 | / | Ta2O5 | RF sputtering | 200 | 120 | 20 | / | |
| Specimen | Ti6Al4V | Ta2O5 | Cu-Ta2O5 |
|---|---|---|---|
| Ecorr (V vs. Ag/AgCl) | −0.42 ± 0.02 | −0.25 ± 0.01 | −0.08 ± 0.01 |
| Icorr (μA/cm2) | 1.07 ± 0.021 | 0.48 ± 0.004 | 0.74 ± 0.005 |
| βa (mV/decade) | 835.8 ± 15 | 373.9 ± 8 | 398.0 ± 4 |
| βc (mV/decade) | 45.1 ± 4 | 51.1 ± 2 | 205.5 ± 4 |
| Rp (Ω·cm−2) | 17.3 ± 0.9 | 55.68 ± 1 | 80 ± 0.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Z.; Wang, Y.; Zhou, Q.; Ding, Z.; Wu, Y.; Zhu, Y.; Shi, W.; He, Q. The Preparation and Properties of Multilayer Cu-MTa2O5 Composite Coatings on Ti6Al4V for Biomedical Applications. Nanomaterials 2019, 9, 1498. https://doi.org/10.3390/nano9101498
Ding Z, Wang Y, Zhou Q, Ding Z, Wu Y, Zhu Y, Shi W, He Q. The Preparation and Properties of Multilayer Cu-MTa2O5 Composite Coatings on Ti6Al4V for Biomedical Applications. Nanomaterials. 2019; 9(10):1498. https://doi.org/10.3390/nano9101498
Chicago/Turabian StyleDing, Zeliang, Yi Wang, Quan Zhou, Ziyu Ding, Yiyong Wu, Yuefang Zhu, Wensong Shi, and Quanguo He. 2019. "The Preparation and Properties of Multilayer Cu-MTa2O5 Composite Coatings on Ti6Al4V for Biomedical Applications" Nanomaterials 9, no. 10: 1498. https://doi.org/10.3390/nano9101498