Flexible and Highly Sensitive Humidity Sensor Based on Sandwich-Like Ag/Fe3O4 Nanowires Composite for Multiple Dynamic Monitoring
Abstract
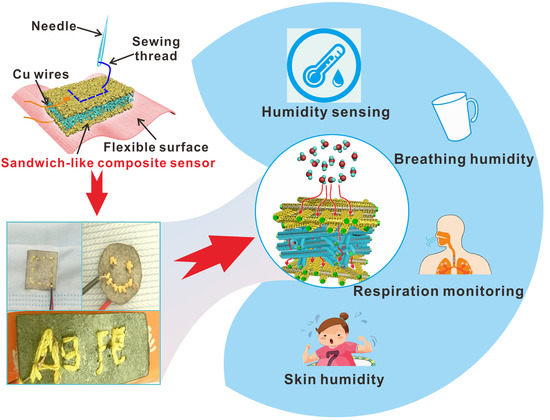
:1. Introduction
2. Experimental Section
2.1. Raw Materials
2.2. Fabrication of Ag@Fe3O4-MS Humidity Sensor
2.3. Characterizations
3. Results and Discussion
3.1. Design and Characterization of Ag@Fe3O4-MS Humidity Sensors
3.2. Humidity Sensing Properties of Ag@Fe3O4-MS
3.3. Health Monitoring
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jeong, Y.R.; Park, H.; Jin, S.W.; Hong, S.Y.; Lee, S.-S.; Ha, J.S. Highly stretchable and sensitive strain sensors using fragmentized graphene foam. Adv. Funct. Mater. 2015, 25, 4228–4236. [Google Scholar] [CrossRef]
- Chen, L.Y.; Tee, B.C.K.; Chortos, A.L.; Schwartz, G.; Tse, V.; Lipomi, D.J.; Wong, H.S.P.; McConnell, M.V.; Bao, Z. Continuous wireless pressure monitoring and mapping with ultra-small passive sensors for health monitoring and critical care. Nat. Commun. 2014, 5, 5028. [Google Scholar] [CrossRef] [PubMed]
- Rothmaier, M.; Selm, B.; Spichtig, S.; Haensse, D. Photonic textiles for pulse oximetry. Opt. Express 2008, 16, 12973–12986. [Google Scholar] [CrossRef] [PubMed]
- Soyoun, J.; Taeksoo, J.; Vijay, K.V. Point-of-care temperature and respiration monitoring sensors for smart fabric applications. Smart Mater. Struct. 2006, 15, 1872. [Google Scholar]
- Devot, S.; Bianchi, A.M.; Naujoka, E.; Mendez, M.O.; Braurs, A.; Cerutti, S. Sleep monitoring through a textile recording system. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 2560–2563. [Google Scholar]
- Frutiger, A.; Muth, J.T.; Vogt, D.M.; Menguec, Y.; Campo, A.; Valentine, A.D.; Walsh, C.J.; Lewis, J.A. Capacitive soft strain sensors via multicore-shell fiber printing. Adv. Mater. 2015, 27, 2440–2446. [Google Scholar] [CrossRef]
- Xue, H.; Yang, Q.; Wang, D.; Luo, W.; Wang, W.; Lin, M.; Liang, D.; Luo, Q. A wearable pyroelectric nanogenerator and self-powered breathing sensor. Nano Energy 2017, 38, 147–154. [Google Scholar] [CrossRef]
- Kao, K.-W.A.; Cheng, C.-J.; Gwo, S.; Yeh, J.A. A semiconductor gas system of healthcare for liver disease detection using ultrathin InN-based sensor. ECS Trans. 2015, 66, 151–157. [Google Scholar] [CrossRef]
- Trung, T.Q.; Duy, L.T.; Ramasundaram, S.; Lee, N.-E. Transparent, stretchable and rapid-response humidity sensor for body-attachable wearable electronics. Nano Res. 2017, 10, 2021–2033. [Google Scholar] [CrossRef]
- Milne, S.D.; Seoudi, I.; Al Hamad, H.; Talal, T.K.; Anoop, A.A.; Allahverdi, N.; Zakaria, Z.; Menzies, R.; Connolly, P. A wearable wound moisture sensor as an indicator for wound dressing change: An observational study of wound moisture and status. Int. Wound J. 2016, 13, 1309–1314. [Google Scholar] [CrossRef]
- Zhao, J.; Li, N.; Yu, H.; Wei, Z.; Liao, M.; Chen, P.; Wang, S.; Shi, D.; Sun, Q.; Zhang, G. Highly sensitive MoS2 humidity sensors array for noncontact sensation. Adv. Mater. 2017, 29, 1702076. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, X.; Cheng, X.-F.; Gao, B.-J.; He, J.-H.; Xu, Q.-F.; Li, H.; Li, N.-J.; Chen, D.-Y.; Lu, J.-M. Surface modification of polysquaraines to sense humidity within a second for breath monitoring. Sens. Actuators B Chem. 2018, 271, 137–146. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Li, P.; Sun, Y.E. Layer-by-layer self-assembly of Co3O4 nanorod-decorated MoS2 nanosheet-based nanocomposite toward high-performance ammonia detection. ACS Appl. Mater. Interfaces 2017, 9, 6462–6471. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Z.; Umar, A.; Wang, Y.; Tian, T.; Shang, Y.; Fan, Y.; Qi, Q.; Xu, D. Supramolecularly modified graphene for ultrafast responsive and highly stable humidity sensor. J Phys. Chem. C 2015, 119, 28640–28647. [Google Scholar] [CrossRef]
- Buvailo, A.I.; Xing, Y.; Hines, J.; Dollahon, N.; Borguet, E. TiO2/LiCl-based nanostructured thin film for humidity sensor applications. ACS Appl. Mater. Interfaces 2011, 3, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Zhao, H.; Fei, T.; Dou, H.; Zhang, T. A guest/host composite of Fe(NO3)3/nanoporous polytriphenylamine assembly for humidity sensor. Sens. Actuators B Chem. 2016, 222, 440–446. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Abu-Thabit, N.; Umar, Y.; Ratemi, E.; Ahmad, A.; Abuilaiwi, F.A. A flexible optical pH sensor based on polysulfone membranes coated with pH-responsive polyaniline nanofibers. Sensors 2016, 16, 986. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.M.; Bruening, M.L. Ultrathin, cross-linked polyimide pervaporation membranes prepared from polyelectrolyte multilayers. J. Membr. Sci. 2005, 248, 161–170. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Z.; Han, S.; Li, C.; Lei, B.; Stewart, M.P.; Tour, J.M.; Zhou, C. Magnetite (Fe3O4) Core−shell nanowires: Synthesis and magnetoresistance. Nano Lett. 2004, 4, 2151–2155. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, D.; Han, S.; Li, C.; Lei, B.; Lu, W.; Fang, J.; Zhou, C. Single crystalline magnetite nanotubes. J. Am. Chem. Soc. 2005, 127, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, T.; Shimazu, H.; Ota, K. Electric conductivity of Fe2SiO4–Fe3O4 spinel solid solutions. Phys. Chem. Miner. 2001, 28, 110–118. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, M.; Zhang, M.; Maimaitiming, A.; Pang, L.; Liang, Y.; Hu, J.; Wu, G. Fe3O4 nanowire arrays on flexible polypropylene substrates for UV and magnetic sensing. ACS Appl. Nano Mater. 2018, 1, 5742–5752. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. 1977, 81, 89–96. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Zong, X.Q.; Wu, Z.L. Ultrahigh-performance impedance humidity sensor based on layer-by-layer self-assembled tin disulfide/titanium dioxide nanohybrid film. Sens. Actuators B Chem. 2018, 266, 52–62. [Google Scholar] [CrossRef]
- Weng, Z.; Qin, J.; Umar, A.A.; Wang, J.; Zhang, X.; Wang, H.; Cui, X.; Li, X.; Zheng, L.; Zhan, Y. Lead-free Cs2BiAgBr6 double perovskite-based humidity sensor with superfast recovery time. Adv. Funct. Mater. 2019, 29, 1902234. [Google Scholar] [CrossRef]
- Zhu, X.L.; Yuan, L.; Liang, G.Z.; Gu, A.J. Unique surface modified aramid fibers with improved flame retardancy, tensile properties, surface activity and UV-resistance through in situ formation of hyperbranched polysiloxane-Ce0.8Ca0.2O1.8 hybrids. J. Mater. Chem. A 2015, 3, 12515–12529. [Google Scholar] [CrossRef]
- Umare, S.S.; Shambharkar, B.H. Synthesis and characterization of polyaniline–Fe3O4 nanocomposite: Electrical conductivity, magnetic, electrochemical studies. Synth. Met. 2010, 160, 1815–1821. [Google Scholar] [CrossRef]
- Virtanen, J.; Ukkonen, L.; Bjorninen, T.; Elsherbeni, A.Z.; Sydänheimo, L. Inkjet-printed humidity sensor for passive UHF RFID systems. IEEE Trans. Instrum. Meas. 2011, 60, 2768–2777. [Google Scholar] [CrossRef]
- Pang, Y.; Jian, J.M.; Tu, T.; Yang, Z.; Ling, J.; Li, Y.X.; Wang, X.F.; Qiao, Y.C.; Tian, H.; Yang, Y.; et al. Wearable humidity sensor based on porous graphene network for respiration monitoring. Biosens. Bioelectron. 2018, 116, 123–129. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, Z.; Wang, D.; Tang, X.; Yan, Y.; Shi, W.; Wang, Y.; Gao, N.; Yao, X.; Dong, H. Construction of high-dispersed Ag/Fe3O4/g-C3N4 photocatalyst by selective photo-deposition and improved photocatalytic activity. Appl. Catal. B Environ. 2016, 182, 115–122. [Google Scholar] [CrossRef]
- Casalbore-Miceli, G.; Yang, M.J.; Camaioni, N.; Mari, C.M.; Li, Y.; Sun, H.; Ling, M. Investigations on the ion transport mechanism in conducting polymer films. Solid State Ion. 2000, 131, 311–321. [Google Scholar] [CrossRef]
- Jain, S.; Chakane, S.; Samui, A.B.; Krishnamurthy, V.N.; Bhoraskar, S.V. Humidity sensing with weak acid-doped polyaniline and its composites. Sens. Actuators B Chem. 2003, 96, 124–129. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Zheng, W.; Wang, W.; Huang, H.; Wang, C.; MacDiarmid, A.G.; Wei, Y. Highly sensitive and stable humidity nanosensors based on LiCl doped TiO2 electrospun nanofibers. J. Am. Chem. Soc. 2008, 130, 5036–5037. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Q.; Lao, C.; Wang, Z.L.; Xie, Z.; Zheng, L. High-sensitivity humidity sensor based on a single SnO2 nanowire. J. Am. Chem. Soc. 2007, 129, 6070–6071. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Xiao, M.; Xiao, Y.; Zou, G.; Hu, L.; Zhao, B.; Liu, L.; Duley, W.W.; Zhou, Y.N. Self-powered, rapid-response and highly flexible humidity sensors based on moisture-dependent voltage generation. ACS Appl. Mater. Interfaces 2019, 11, 14249–14255. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, D.; Kwak, B.; Lee, H.-S.; Lee, S.; Yoo, B. Synthesis of self-bridged ZnO nanowires and their humidity sensing properties. Sens. Actuators B Chem. 2018, 268, 293–298. [Google Scholar] [CrossRef]
- Liu, L.X.; Chen, W.; Zhang, H.B.; Wang, Q.W.; Guan, F.; Yu, Z.Z. Flexible and multifunctional silk textiles with biomimetic leaf-like mxene/silver nanowire nanostructures for electromagnetic interference shielding, humidity monitoring and self-derived hydrophobicity. Adv. Funct. Mater. 2019. [Google Scholar] [CrossRef]







| Substrate | Sensor Materials | Flexible or Rigid | Free-Cutting | Detection Range (% RH) | Reference |
|---|---|---|---|---|---|
| Si | SnO2 NWs | rigid | no | 30–85 | [35] |
| poly(ethylene terephthalate) | TiO2 NWs | flexible | no | 20–90 | [36] |
| SiO/Si | ZnO NWs | rigid | no | 10–90 | [37] |
| Polyurethane | Ag NWs | flexible | no | 0–80 | [38] |
| Polypropylene | Ag/Fe3O4 NWs | flexible | free cutting/embroidery | 11–95 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wang, M.; Zhang, M.; Qiu, L.; Liu, Y.; Zhang, W.; Zhang, Y.; Hu, J.; Wu, G. Flexible and Highly Sensitive Humidity Sensor Based on Sandwich-Like Ag/Fe3O4 Nanowires Composite for Multiple Dynamic Monitoring. Nanomaterials 2019, 9, 1399. https://doi.org/10.3390/nano9101399
Zhang M, Wang M, Zhang M, Qiu L, Liu Y, Zhang W, Zhang Y, Hu J, Wu G. Flexible and Highly Sensitive Humidity Sensor Based on Sandwich-Like Ag/Fe3O4 Nanowires Composite for Multiple Dynamic Monitoring. Nanomaterials. 2019; 9(10):1399. https://doi.org/10.3390/nano9101399
Chicago/Turabian StyleZhang, Maojiang, Minglei Wang, Mingxing Zhang, Long Qiu, Yinjie Liu, Wenli Zhang, Yumei Zhang, Jiangtao Hu, and Guozhong Wu. 2019. "Flexible and Highly Sensitive Humidity Sensor Based on Sandwich-Like Ag/Fe3O4 Nanowires Composite for Multiple Dynamic Monitoring" Nanomaterials 9, no. 10: 1399. https://doi.org/10.3390/nano9101399