Facile Synthesis of Well-Dispersed Ni2P on N-Doped Nanomesh Carbon Matrix as a High-Efficiency Electrocatalyst for Alkaline Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of NC
2.3. Synthesis of Bulk Ni2P
2.4. Synthesis of Ni2P/NC
2.5. Characterizations
2.6. Electrochemical Measurements
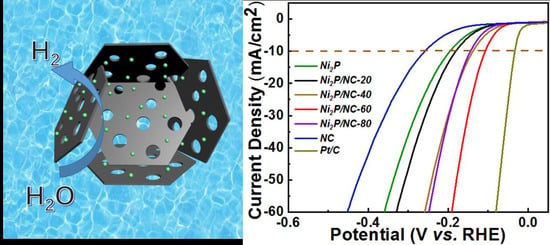
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rausch, B.; Symes, M.D.; Chisholm, G.; Cronin, L. Decoupled catalytic hydrogen evolution from a molecular metal oxide redox mediator in water splitting. Science 2014, 345, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Liu, X.Y.; Liu, Y.; Yang, G.W. Active Pore-Edge Engineering of Single-Layer Niobium Diselenide Porous Nanosheets Electrode for Hydrogen Evolution. Nanomaterials 2019, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, D.; Yang, H.H.; Huang, H.; Luo, Q.; Huang, Y.F.; Yu, X.F.; Chu, P.K. Modification of Layered Graphitic Carbon Nitride by Nitrogen Plasma for Improved Electrocatalytic Hydrogen Evolution. Nanomaterials 2019, 9, 568. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Lin, J.; Xu, H.; Tong, Y.X.; Li, G.R. Ni2P–CoP hybrid nanosheet arrays supported on carbon cloth as an efficient flexible cathode for hydrogen evolution. J. Mater. Chem. 2016, 4, 16992–16999. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Colli, A.N.; Girault, H.H.; Battistel, A. Non-Precious Electrodes for Practical Alkaline Water Electrolysis. Materials 2019, 12, 1336. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Guo, Y.; She, S.; Miao, S.; Ni, M.; Zhou, W.; Liu, M.; Shao, Z. Bigger is Surprisingly Better: Agglomerates of Larger RuP Nanoparticles Outperform Benchmark Pt Nanocatalysts for the Hydrogen Evolution Reaction. Adv. Mater. 2018, 30, e1800047. [Google Scholar] [CrossRef]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.K.; Liu, L.M.; et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef]
- Pu, Z.; Amiinu, I.S.; Kou, Z.; Li, W.; Mu, S. RuP2-Based Catalysts with Platinum-like Activity and Higher Durability for the Hydrogen Evolution Reaction at All pH Values. Angew. Chem. Int. Ed. 2017, 56, 11559–11564. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, X.; Ning, S.; Kang, X.; Chen, S. Supported Heterostructured MoC/Mo2C Nanoribbons and Nanoflowers as Highly Active Electrocatalysts for Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2019, 7, 8458–8465. [Google Scholar] [CrossRef]
- Huang, Y.; Ge, J.; Hu, J.; Zhang, J.; Hao, J.; Wei, Y. Nitrogen-Doped Porous Molybdenum Carbide and Phosphide Hybrids on a Carbon Matrix as Highly Effective Electrocatalysts for the Hydrogen Evolution Reaction. Adv. Energy Mater. 2018, 8, e1701601. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Yang, L.; Yang, F.; Cheng, G.; Luo, W. Nest-like NiCoP for Highly Efficient Overall Water Splitting. ACS Catal. 2017, 7, 4131–4137. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ma, M.; Ren, X.; Liu, Z.; Du, G.; Asiri, A.M.; Sun, X. A Mn-doped Ni2P nanosheet array: An efficient and durable hydrogen evolution reaction electrocatalyst in alkaline media. Chem. Commun. 2017, 53, 11048–11051. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Rodriguez, J.A. Catalysts for Hydrogen Evolution from the [NiFe] Hydrogenase to the Ni2P (001) Surface: The Importance of Ensemble Effect. J. Am. Chem. Soc. 2005, 127, 14871–14878. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Z.; Zhao, H.; Huang, H.; Jiao, H.; Du, Y. MOF-derived porous Ni2P nanosheets as novel bifunctional electrocatalysts for the hydrogen and oxygen evolution reactions. J. Mater. Chem. 2018, 6, 18720–18727. [Google Scholar] [CrossRef]
- Jin, X.; Li, J.; Cui, Y.; Liu, X.; Wang, K.; Zhou, Y.; Yang, W.; Zhang, X.; Zhang, C.; Jiang, X.; et al. In-situ synthesis of porous Ni2P nanosheets for efficient and stable hydrogen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 5739–5747. [Google Scholar] [CrossRef]
- Wang, X.; Kolen’ko, Y.V.; Bao, X.Q.; Kovnir, K.; Liu, L. One-Step Synthesis of Self-Supported Nickel Phosphide Nanosheet Array Cathodes for Efficient Electrocatalytic Hydrogen Generation. Angew. Chem. Int. Ed. 2015, 54, 8188–8192. [Google Scholar] [CrossRef]
- Yan, L.; Jiang, H.; Wang, Y.; Li, L.; Gu, X.; Dai, P.; Liu, D.; Tang, S.F.; Zhao, G.; Zhao, X.; et al. One-step and scalable synthesis of Ni2P nanocrystals encapsulated in N, P-codoped hierarchically porous carbon matrix using a bipyridine and phosphonate linked nickel metal–organic framework as highly efficient electrocatalysts for overall water splitting. Electrochim. Acta 2019, 297, 755–766. [Google Scholar] [CrossRef]
- Zhou, Q.; Shen, Z.; Zhu, C.; Li, J.; Ding, Z.; Wang, P.; Pan, F.; Zhang, Z.; Ma, H.; Wang, S.; et al. Nitrogen-Doped CoP Electrocatalysts for Coupled Hydrogen Evolution and Sulfur Generation with Low Energy Consumption. Adv. Mater. 2018, 30, e1800140. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Li, X.; Li, C.; Zhang, R.; Zhang, Y.; Zhu, H. Sponge-like nickel phosphide–carbon nanotube hybrid electrodes for efficient hydrogen evolution over a wide pH range. Nano Res. 2016, 10, 415–425. [Google Scholar] [CrossRef]
- Pan, Y.; Hu, W.; Liu, D.; Liu, Y.; Liu, C. Carbon nanotubes decorated with nickel phosphide nanoparticles as efficient nanohybrid electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. 2015, 3, 13087–13094. [Google Scholar] [CrossRef]
- Jin, H.Y.; Wang, J.; Su, D.F.; Wei, Z.Z.; Pang, Z.F.; Wang, Y. In situ Cobalt–Cobalt Oxide/N-Doped Carbon Hybrids as Superior Bifunctional Electrocatalysts for Hydrogen and Oxygen Evolution. J. Am. Chem. Soc. 2015, 137, 2688–2694. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yang, Y.; Xia, G.; Chen, J.; Jiang, P.; Chen, Q. Corrigendum: Ruthenium-cobalt nanoalloys encapsulated in nitrogen-doped graphene as active electrocatalysts for producing hydrogen in alkaline media. Nat. Commun. 2017, 8, 16028. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Zhang, Y.; Jiang, W.J.; Zhang, X.; Dai, Z.; Wan, L.J.; Hu, J.S. Pomegranate-like N, P-Doped Mo2C@C Nanospheres as Highly Active Electrocatalysts for Alkaline Hydrogen Evolution. ACS Nano 2016, 10, 8851–8860. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Hou, R.; Liang, Z.; Qi, R.; He, T.; Yan, Y.; Qi, K.; Liu, H.; Feng, G.; Xia, B.Y. Chainmail catalyst of ultrathin P-doped carbon shell-encapsulated nickel phosphides on graphene towards robust and efficient hydrogen generation. J. Mater. Chem. 2018, 6, 24107–24113. [Google Scholar] [CrossRef]
- Cao, L.; Luo, Q.; Liu, W.; Lin, Y.; Liu, X.; Cao, Y.; Zhang, W.; Wu, Y.; Yang, J.; Yao, T.; et al. Identification of single-atom active sites in carbon-based cobalt catalysts during electrocatalytic hydrogen evolution. Nat. Catal. 2018, 2, 134–141. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, N.; Chen, Y.; Lin, Y.; Li, Y.; Liu, Y.; Liu, C. Nickel phosphide nanoparticles-nitrogen-doped graphene hybrid as an efficient catalyst for enhanced hydrogen evolution activity. J. Power Sources 2015, 297, 45–52. [Google Scholar] [CrossRef]
- Li, Y.; Cai, P.; Ci, S.; Wen, Z. Strongly Coupled 3D Nanohybrids with Ni2P/Carbon Nanosheets as pH-Universal Hydrogen Evolution Reaction Electrocatalysts. ChemElectroChem 2017, 4, 340–344. [Google Scholar] [CrossRef]
- Yang, F.; Wang, M.; Liu, W.; Yang, B.; Wang, Y.; Luo, J.; Tang, Y.; Hou, L.; Li, Y.; Li, Z.; et al. Atomically dispersed Ni as the active site towards selective hydrogenation of nitroarenes. Green Chem. 2019, 21, 704–711. [Google Scholar] [CrossRef]
- Yang, F.; Fan, X.; Wang, C.; Yang, W.; Hou, L.; Xu, X.; Feng, A.; Dong, S.; Chen, K.; Wang, Y.; et al. P-doped nanomesh graphene with high-surface-area as an efficient metal-free catalyst for aerobic oxidative coupling of amines. Carbon 2017, 121, 443–451. [Google Scholar] [CrossRef]
- Ma, X.; Ning, G.; Qi, C.; Xu, C.; Gao, J. Phosphorus and nitrogen dual-doped few-layered porous graphene: A high-performance anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 14415–14422. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, C.; Dong, S.; Chi, C.; Jia, X.; Zhang, L.; Li, Y. Plasma synthesis of Pd/PdO supported on porous graphene as electrocatalyst for methanol oxidation. Mater. Lett. 2016, 174, 192–196. [Google Scholar] [CrossRef]
- Guan, Q.; Han, F.; Li, W. Catalytic performance and deoxygenation path of methyl palmitate on Ni2P/SiO2 synthesized using the thermal decomposition of nickel hypophosphite. RSC Adv. 2016, 6, 31308–31315. [Google Scholar] [CrossRef]
- Yang, F.; Chi, C.; Wang, C.; Wang, Y.; Li, Y. High graphite N content in nitrogen-doped graphene as an efficient metal-free catalyst for reduction of nitroarenes in water. Green Chem. 2016, 18, 4254–4262. [Google Scholar] [CrossRef]
- Wei, C.; Rao, R.R.; Peng, J.; Huang, B.; Stephens, I.E.L.; Risch, M.; Xu, Z.J.; Shao-Horn, Y. Recommended Practices and Benchmark Activity for Hydrogen and Oxygen Electrocatalysis in Water Splitting and Fuel Cells. Adv. Mater. 2019, 31, e1806296. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Feng, L.; Liu, C.; Xing, W.; Hu, X. Ni2P enhances the activity and durability of the Pt anode catalyst in direct methanol fuel cells. Energy Environ. Sci. 2014, 7, 1628–1632. [Google Scholar] [CrossRef]
- Wang, W.; An, T.; Li, G.; Xia, D.; Zhao, H.; Yu, J.C.; Wong, P.K. Earth-abundant Ni2P/g-C3N4 lamellar nanohydrids for enhanced photocatalytic hydrogen evolution and bacterial inactivation under visible light irradiation. Appl. Catal. B Environ. 2017, 217, 570–580. [Google Scholar] [CrossRef]
- Chang, J.; Feng, L.; Liu, C.; Xing, W.; Hu, X. An effective Pd-Ni2P/C anode catalyst for direct formic acid fuel cells. Angew. Chem. Int. Ed. 2014, 53, 122–126. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Y.; Cheng, G.; Chen, S.; Luo, W. Ultrathin Nitrogen-Doped Carbon Coated with CoP for Efficient Hydrogen Evolution. ACS Catal. 2017, 7, 3824–3831. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.X.; Wu, D.L.; Xia, W.; Jia, D.Z. Interaction between nitrogen and sulfur in co-doped graphene and synergetic effect in supercapacitor. Sci. Rep. 2015, 5, 9591. [Google Scholar] [CrossRef] [PubMed]
- He, S.; He, S.; Bo, X.; Wang, Q.; Zhan, F.; Wang, Q.; Zhao, C. Porous Ni2P/C microrods derived from microwave-prepared MOF-74-Ni and its electrocatalysis for hydrogen evolution reaction. Mater. Lett. 2018, 231, 94–97. [Google Scholar] [CrossRef]
- Dong, C.; Guo, L.; He, Y.; Chen, C.; Qian, Y.; Chen, Y.; Xu, L. Sandwich-like Ni2P nanoarray/nitrogen-doped graphene nanoarchitecture as a high-performance anode for sodium and lithium ion batteries. Energy Storage Mater. 2018, 15, 234–241. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Xu, X.X.; Hu, B.C.; Liang, H.W.; Lin, Y.; Chen, L.F.; Yu, S.H. Iron Carbide Nanoparticles Encapsulated in Mesoporous Fe-N-Doped Carbon Nanofibers for Efficient Electrocatalysis. Angew. Chem. Int. Ed. 2015, 54, 8179–8183. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Guo, S.; Pinna, N. Graphene/N-doped carbon sandwiched nanosheets with ultrahigh nitrogen doping for boosting lithium-ion batteries. J. Mater. Chem. 2016, 4, 1423–1431. [Google Scholar] [CrossRef]
- Ren, G.; Lu, X.; Li, Y.; Zhu, Y.; Dai, L.; Jiang, L. Porous Core-Shell Fe3C Embedded N-doped Carbon Nanofibers as an Effective Electrocatalysts for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 4118–4125. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.C.; Liu, Y.Q.; Wang, Y.; Zhang, H.L.; Huang, L.P.; Yu, G. Synthesis of N-Doped Graphene by Chemical Vapor Deposition and Its Electrical Properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wang, D.; Jiang, Z.; Zhang, J.; Shi, S.; Jiang, Z.J.; Liu, M. Amine group induced high activity of highly torn amine functionalized nitrogen-doped graphene as the metal-free catalyst for hydrogen evolution reaction. Carbon 2018, 138, 169–178. [Google Scholar] [CrossRef]
- Zhou, Z.; Wei, L.; Wang, Y.; Karahan, H.E.; Chen, Z.; Lei, Y.; Chen, X.; Zhai, S.; Liao, X.; Chen, Y. Hydrogen evolution reaction activity of nickel phosphide is highly sensitive to electrolyte pH. J. Mater. Chem. 2017, 5, 20390–20397. [Google Scholar] [CrossRef]
- Dai, X.; Song, H.; Yan, Z.; Li, F.; Chen, Y.; Wang, X.; Yuan, D.; Zhang, J.; Wang, Y. Effect of preparation temperature on the structures and hydrodeoxygenation performance of Ni2P/C catalysts prepared by decomposition of hypophosphites. New J. Chem. 2018, 42, 19917–19923. [Google Scholar] [CrossRef]
- Xue, Y.; Guan, Q.; Li, W. Synthesis of bulk and supported nickel phosphide using microwave radiation for hydrodeoxygenation of methyl palmitate. RSC Adv. 2015, 5, 53623–53628. [Google Scholar] [CrossRef]
- Feng, L.; Vrubel, H.; Bensimon, M.; Hu, X. Easily-prepared dinickel phosphide (Ni2P) nanoparticles as an efficient and robust electrocatalyst for hydrogen evolution. Phys. Chem. Chem. Phys. 2014, 16, 5917–5921. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ouyang, B.; Xu, J.; Chen, S.; Rawat, R.S.; Fan, H.J. 3D Porous Hierarchical Nickel-Molybdenum Nitrides Synthesized by RF Plasma as Highly Active and Stable Hydrogen-Evolution-Reaction Electrocatalysts. Adv. Energy Mater. 2016, 6, e1600221. [Google Scholar] [CrossRef]
- Lei, C.; Wang, Y.; Hou, Y.; Liu, P.; Yang, J.; Zhang, T.; Zhuang, X.; Chen, M.; Yang, B.; Lei, L.; et al. Efficient alkaline hydrogen evolution on atomically dispersed Ni–Nx Species anchored porous carbon with embedded Ni nanoparticles by accelerating water dissociation kinetics. Energy Environ. Sci. 2019, 12, 149–156. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Liu, P.; Liao, Z.; Liu, S.; Zhuang, X.; Chen, M.; Zschech, E.; Feng, X. Efficient hydrogen production on MoNi4 electrocatalysts with fast water dissociation kinetics. Nat. Commun. 2017, 8, 15437. [Google Scholar] [CrossRef] [PubMed]
- Long, G.F.; Wan, K.; Liu, M.Y.; Liang, Z.X.; Piao, J.H.; Tsiakaras, P. Active sites and mechanism on nitrogen-doped carbon catalyst for hydrogen evolution reaction. J. Catal. 2017, 348, 151–159. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Yan, Z.; Chen, X.; Cheng, F.; Weiss, P.S.; Chen, J. Porous Multishelled Ni2P Hollow Microspheres as an Active Electrocatalyst for Hydrogen and Oxygen Evolution. Chem. Mater. 2017, 29, 8539–8547. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, R.; Lu, W.; Wang, Z.; Liu, D.; Hao, S.; Du, G.; Asiri, A.M.; Sun, X. Energy-Saving Electrolytic Hydrogen Generation: Ni2P Nanoarray as a High-Performance Non-Noble-Metal Electrocatalyst. Angew. Chem. Int. Ed. 2017, 56, 842–846. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Liu, H.; Suen, N.T.; Yu, X.; Feng, L. Electrochemical Hydrogen Evolution Reaction Efficiently Catalyzed by Ru2P Nanoparticles. ChemSusChem 2018, 11, 2724–2729. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Huang, S.; Zhang, B.; Hou, L.; Ding, Y.; Bao, W.; Xu, C.; Yang, W.; Li, Y. Facile Synthesis of Well-Dispersed Ni2P on N-Doped Nanomesh Carbon Matrix as a High-Efficiency Electrocatalyst for Alkaline Hydrogen Evolution Reaction. Nanomaterials 2019, 9, 1022. https://doi.org/10.3390/nano9071022
Yang F, Huang S, Zhang B, Hou L, Ding Y, Bao W, Xu C, Yang W, Li Y. Facile Synthesis of Well-Dispersed Ni2P on N-Doped Nanomesh Carbon Matrix as a High-Efficiency Electrocatalyst for Alkaline Hydrogen Evolution Reaction. Nanomaterials. 2019; 9(7):1022. https://doi.org/10.3390/nano9071022
Chicago/Turabian StyleYang, Fan, Shuo Huang, Bing Zhang, Liqiang Hou, Yi Ding, Weijie Bao, Chunming Xu, Wang Yang, and Yongfeng Li. 2019. "Facile Synthesis of Well-Dispersed Ni2P on N-Doped Nanomesh Carbon Matrix as a High-Efficiency Electrocatalyst for Alkaline Hydrogen Evolution Reaction" Nanomaterials 9, no. 7: 1022. https://doi.org/10.3390/nano9071022