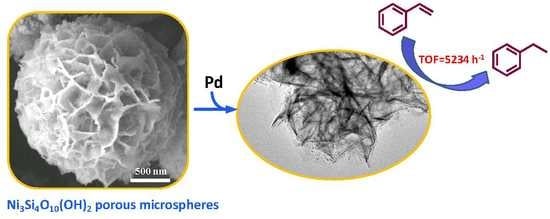
Synthesis of Ni3Si4O10(OH)2 Porous Microspheres as Support of Pd Catalyst for Hydrogenation Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of NiSi-PMs
2.2. Preparation of Pd/NiSi-PMs Catalyst
2.3. Characterization
2.4. Evaluation of Catalytic Activity
3. Results and Discussion
3.1. Synthesis of NiSi-PMs
3.2. Performance of the Pd/NiSi-PMs Catalyst for the Hydrogenation of Styrene
3.3. H2-TPR and H2-TPD Analysis of the Catalysts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feliczak-Guzik, A.; Szczyglewska, P.; Nowak, I. The effect of metal (Nb, Ru, Pd, Pt) supported on SBA-16 on the hydrodeoxygenation reaction of phenol. Catal. Today 2019, 325, 61–67. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Le, X.; Zhang, W.; Gu, H.; Xue, R.; Ma, J. Catalysis of the hydro-dechlorination of 4-chlorophenol by Pd(0)-modified MCM-48 mesoporous microspheres with an ultra-high surface area. New J. Chem. 2015, 39, 4519–4525. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Xia, C.; Li, F. Nitrogen-functionalized ordered mesoporous carbons as multifunctional supports of ultrasmall Pd nanoparticles for hydrogenation of phenol. ACS Catal. 2013, 3, 2440–2448. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Zhao, Y.; Liu, C.J. Recent progresses in the size and structure control of MOF supported noble metal catalysts. Catal. Today 2016, 263, 61–68. [Google Scholar] [CrossRef]
- Gao, D.; Wang, Z.; Wang, C.; Wang, L.; Chi, Y.; Wang, M.; Zhang, J.; Wu, C.; Gu, Y.; Wang, H.; et al. CrPd nanoparticles on NH2-functionalized metal-organic framework as a synergistic catalyst for efficient hydrogen evolution from formic acid. Chem. Eng. J. 2019, 361, 953–959. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Q.; Xiong, J.; Li, J.; Liu, J.; Zhao, Z.; Hao, S. Efficient catalysts of supported PtPd nanoparticles on 3D ordered macroporous TiO2 for soot combustion: Synergic effect of Pt-Pd binary components. Catal. Today 2019, 327, 143–153. [Google Scholar] [CrossRef]
- Xie, J.; Yao, X.; Cheng, Q.; Madden, I.P.; Dornath, P.; Chang, C.C.; Fan, W.; Wang, D. Three dimensionally ordered mesoporous carbon as a stable, high-performance Li–O2 battery cathode. Angew. Chem. Int. Ed. 2015, 54, 4299–4303. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, Q.; Li, J. Template synthesis of aligned carbon nanotube arrays using glucose as a carbon source: Pt decoration of inner and outer nanotube surfaces for fuel-cell catalysts. Adv. Funct. Mater. 2008, 18, 959–964. [Google Scholar] [CrossRef]
- Cao, J.; Hu, Y.; Chen, L.; Xu, J.; Chen, Z. Nitrogen-doped carbon quantum dot/graphene hybrid nanocomposite as an efficient catalyst support for the oxygen reduction reaction. Int. J. Hydrogen Energy 2017, 42, 2931–2942. [Google Scholar] [CrossRef]
- Ke, C.; Li, M.; Fan, G.; Yang, L.; Li, F. Pt Nanoparticles supported on nitrogen-doped-carbon-decorated CeO2 for base-free aerobic oxidation of 5-hydroxymethylfurfural. Chem. Asian J. 2018, 13, 2714–2722. [Google Scholar] [CrossRef]
- Onn, T.M.; Monai, M.; Dai, S.; Fonda, E.; Montini, T.; Pan, X.; Graham, G.W.; Fornasiero, P.; Gorte, R.J. Smart Pd Catalyst with Improved Thermal Stability Supported on High-Surface-Area LaFeO3 Prepared by Atomic Layer Deposition. J. Am. Chem. Soc. 2018, 140, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 2002, 418, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhu, X.; Abney, C.W.; Liu, X.; Foo, G.S.; Wu, Z.; Li, M.; Meyer, H.M.; Brown, S.; Mahurin, S.M.; et al. Toward the Design of a Hierarchical Perovskite Support: Ultra-Sintering-Resistant Gold Nanocatalysts for CO Oxidation. ACS Catal. 2017, 7, 3388–3393. [Google Scholar] [CrossRef]
- Sivaiah, M.V.; Petit, S.; Beaufort, M.F.; Eyidi, D.; Barrault, J.; Batiot-Dupeyrat, C.; Valange, S. Nickel based catalysts derived from hydrothermally synthesized 1:1 and 2:1 phyllosilicates as precursors for carbon dioxide reforming of methane. Micropor. Mesopor. Mater. 2011, 140, 69–80. [Google Scholar] [CrossRef]
- Yan, L.; Liu, X.; Deng, J.; Fu, Y. Molybdenum modified nickel phyllosilicates as a high performance bifunctional catalyst for deoxygenation of methyl palmitate to alkanes under mild conditions. Green Chem. 2017, 19, 4600–4609. [Google Scholar] [CrossRef]
- Ashok, J.; Bian, Z.; Wang, Z.; Kawi, S. Ni-phyllosilicate structure derived Ni–SiO2–MgO catalysts for bi-reforming applications: Acidity, basicity and thermal stability. Catal. Sci. Technol. 2018, 8, 1730–1742. [Google Scholar] [CrossRef]
- Yang, M.; Jin, P.; Fan, Y.; Huang, C.; Zhang, N.; Weng, W.; Chen, M.; Wan, H. Ammonia-assisted synthesis towards a phyllosilicate-derived highly-dispersed and long-lived Ni/SiO2 catalyst. Catal. Sci. Technol. 2015, 5, 5095–5099. [Google Scholar] [CrossRef]
- Bian, Z.; Kawi, S. Highly carbon-resistant Ni–Co/SiO2 catalysts derived from phyllosilicates for dry reforming of methane. J. CO2 Util. 2017, 18, 345–352. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Q.; Li, J.; Zhuang, Y.; He, Y.; Bai, B.; Wang, X. Ni3Si2O5(OH)4 multi-walled nanotubes with tunable magnetic properties and their application as anode materials for lithium batteries. Nano Res. 2011, 4, 882–890. [Google Scholar] [CrossRef]
- McDonald, A.; Scott, B.; Villemure, G. Hydrothermal preparation of nanotubular particles of a 1:1 nickel phyllosilicate. Micropor. Mesopor. Mater. 2009, 120, 263–266. [Google Scholar] [CrossRef]
- White, R.D.; Bavykin, D.V.; Walsh, F.C. Morphological control of synthetic Ni3Si2O5(OH)4 nanotubes in an alkaline hydrothermal environment. J. Mater. Chem. A 2013, 1, 548–556. [Google Scholar] [CrossRef]
- Guo, Z.; Du, F.; Li, G.; Cui, Z. Controlled synthesis of mesoporous SiO2/Ni3Si2O5(OH)4 core-shell microspheres with tunable chamber structures via a self-template method. Chem. Commun. 2008, 25, 2911–2913. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Guo, Z.; Sun, T.; Du, F. Controlled synthesis and catalytic properties of mesoporous nickel–silica core–shell microspheres with tunable chamber structures. Mater. Res. Bull. 2012, 47, 2344–2348. [Google Scholar] [CrossRef]
- Dong, F.; Xiong, T.; Wang, R.; Sun, Y.; Jiang, Y. Growth mechanism and photocatalytic activity of self-organized N-doped (BiO)2CO3 hierarchical nanosheet microspheres from bismuth citrate and urea. Dalton Trans. 2014, 43, 6631–6642. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, L.; Sun, P.; Zhai, P.; Chen, X.; Zhang, H.; Zhang, Z.; Zhu, W. Novel application of red mud: Facile hydrothermal-thermal conversion synthesis of hierarchical porous AlOOH and Al2O3 microspheres as adsorbents for dye removal. Chem. Eng. J. 2017, 321, 622–634. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, R.; Song, S.; Xing, Y. Synthesis of flower-like nickel oxide/nickel silicate nanocomposites and their enhanced electrochemical performance as anode materials for lithium batteries. Mater. Lett. 2013, 93, 5–8. [Google Scholar] [CrossRef]
- Jin, R.; Sun, S.; Yang, Y.; Xing, Y.; Yu, D.; Yu, X.; Song, S. Size-dependent catalytic properties of Au nanoparticles supported on hierarchical nickel silicate nanostructures. Dalton Trans. 2013, 42, 7888–7893. [Google Scholar] [CrossRef]
- Harraz, F.A.; El-Hout, S.E.; Killa, H.M.; Ibrahim, I.A. Palladium nanoparticles stabilized by polyethylene glycol: Efficient, recyclable catalyst for hydrogenation of styrene and nitrobenzene. J. Catal. 2012, 286, 184–192. [Google Scholar] [CrossRef]
- Pan, Y.; Ma, D.; Liu, H.; Wu, H.; He, D.; Li, Y. Uncoordinated carbonyl groups of MOFs as anchoring sites for the preparation of highly active Pd nano-catalysts. J. Mater. Chem. 2012, 22, 10834–10839. [Google Scholar] [CrossRef]
- Hwang, C.B.; Fu, Y.S.; Lu, Y.L.; Jang, S.W.; Chou, P.T.; Wang, C.R.C.; Yu, S.J. Synthesis, characterization, and highly efficient catalytic reactivity of suspended palladium nanoparticles. J. Catal. 2000, 195, 336–341. [Google Scholar] [CrossRef]
- Islam, S.M.; Roy, A.S.; Mondal, P.; Salam, N. Selective hydrogenation and Suzuki cross-coupling reactions of various organic substrates using a reusable polymer-anchored palladium(II) Schiff base complex. Appl. Organomet. Chem. 2012, 26, 625–634. [Google Scholar] [CrossRef]
- Yang, S.; Cao, C.; Sun, Y.; Huang, P.; Wei, F.; Song, W. Nanoscale magnetic stirring bars for heterogeneous catalysis in microscopic systems. Angew. Chem. Int. Ed. 2015, 54, 2661–2664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Liu, X.; Ma, X.; Zhu, W. Hydrothermal synthesis of pure-phase hierarchical porous hexagonal WO3 microspheres as highly efficient support for Pd catalyst for hydrogenation. Particuology 2018, 41, 126–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Quek, X.Y.; Wu, L.; Guan, Y.; Hensen, E.J. Palladium nanoparticles entrapped in polymeric ionic liquid microgels as recyclable hydrogenation catalysts. J. Mol. Catal. A Chem. 2013, 379, 53–58. [Google Scholar] [CrossRef]
- Imran, M.; Yousaf, A.B.; Zhou, X.; Jiang, Y.F.; Yuan, C.Z.; Zeb, A.; Jiang, N.; Xu, A.W. Pd/TiO Nanocatalyst with strong metal–support interaction for highly efficient durable heterogeneous hydrogenation. J. Phys. Chem. C 2017, 121, 1162–1170. [Google Scholar] [CrossRef]
- Ullah, N.; Imran, M.; Liang, K.; Yuan, C.Z.; Zeb, A.; Jiang, N.; Qazi, U.Y.; Sahar, S.; Xu, A.W. Highly dispersed ultra-small Pd nanoparticles on gadolinium hydroxide nanorods for efficient hydrogenation reactions. Nanoscale 2017, 9, 13800–13807. [Google Scholar] [CrossRef]
- Horiuti, I.; Polanyi, M. Exchange reactions of hydrogen on metallic catalysts. Trans. Faraday Soc. 1934, 30, 1164–1172. [Google Scholar] [CrossRef]
- Wilde, M.; Fukutani, K.; Ludwig, W.; Brandt, B.; Fischer, J.H.; Schauermann, S.; Freund, H.J. Influence of carbon deposition on the hydrogen distribution in Pd nanoparticles and their reactivity in olefin hydrogenation. Angew. Chem. Int. Ed. 2008, 47, 9289–9293. [Google Scholar] [CrossRef]
- Padmasri, A.H.; Venugopal, A.; Siva Kumar, V.; Shashikala, V.; Nagaraja, B.M.; Seetharamulu, P.; Sreedhar, B.; David Raju, B.; Kanta Rao, P.; Rama Rao, K.S. Role of hydrotalcite precursors as supports for Pd catalysts in hydrodechlorination of CCl2F2. J. Mol. Catal. A Chem. 2004, 223, 329–337. [Google Scholar] [CrossRef]
- Fagherazzi, G.; Benedetti, A.; Polizzi, S.; Di Mario, A.; Pinna, F.; Signoretto, M.; Pernicone, N. Structural investigation on the stoichiometry of β-PdHx in Pd/SiO2 catalysts as a function of metal dispersion. Catal. Lett. 1995, 32, 293–303. [Google Scholar] [CrossRef]







| Catalyst | Pd Loading (wt.%) | Size of Pd Nanoparticles (nm) | H2 Pressure (atm) | Solvent | TOF (h−1) | Reference |
|---|---|---|---|---|---|---|
| Pd/ZIF-8 | 1 | NA a | 1 b | None | 307 | [29] |
| Pd/Tm-MOF | 1 | NA a | 1 b | None | 703 | [29] |
| Pd/C | 10 | NA a | 40.8 b | Ethyl acetate | 537 | [30] |
| Pd/PEG | 3.75 | 5 | 1 b | Ethanol | 660 | [28] |
| polymer anchored palladium | 12.1 | NA a | 1 b | DMF | 766 | [31] |
| Fe3O4-NC-PZS-Pd | 3.6 | 3.5 ± 1.5 | 1 b | Ethanol | 1792 | [32] |
| Pd/h-WO3 | 1 | 6 | 1 b | Ethanol | 3050 | [33] |
| Pd/PIL-Br | 10 | 3.3 | 10 b | Methanol | 4800 | [34] |
| Pd/TiO | 1 | 10 | 1 c | Ethanol | 4838 | [35] |
| Pd/NiSi-PMs | 1 | 8.7 ± 2.0 | 1 b | Ethanol | 5234 | This work |
| Pd/Gd(OH)3 | 0.95 | 2-3 | 1 c | Ethanol | 6159 | [36] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Liu, C.; Ma, X.; Zhu, W.; Lv, X.; Zhang, H. Synthesis of Ni3Si4O10(OH)2 Porous Microspheres as Support of Pd Catalyst for Hydrogenation Reaction. Nanomaterials 2019, 9, 998. https://doi.org/10.3390/nano9070998
Wang T, Liu C, Ma X, Zhu W, Lv X, Zhang H. Synthesis of Ni3Si4O10(OH)2 Porous Microspheres as Support of Pd Catalyst for Hydrogenation Reaction. Nanomaterials. 2019; 9(7):998. https://doi.org/10.3390/nano9070998
Chicago/Turabian StyleWang, Tingting, Chenyuan Liu, Xinxin Ma, Wancheng Zhu, Xiaoxia Lv, and Heng Zhang. 2019. "Synthesis of Ni3Si4O10(OH)2 Porous Microspheres as Support of Pd Catalyst for Hydrogenation Reaction" Nanomaterials 9, no. 7: 998. https://doi.org/10.3390/nano9070998