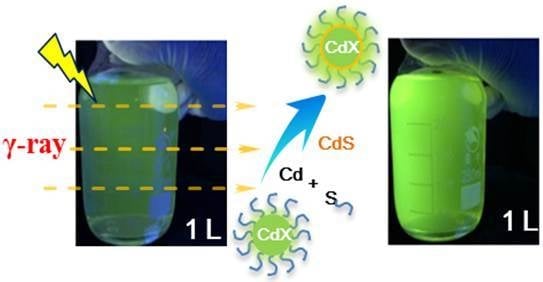
γ-Radiation Enhanced Luminescence of Thiol-Capped Quantum Dots in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MPA-CdTe, MPA-CdSe and Cys-CdTe QDs
2.3. Irradiation of MPA-CdTe, MPA-CdSe and Cys-CdTe QDs in Solution
2.4. Characterization
3. Results and Discussion
3.1. Photoluminescence Properties of As-Prepared QDs Before and After Irradiation
3.2. Morphology and Structure of As-Prepared QDs Before and After γ-Irradiation
3.3. Mechanism of γ-Radiation-Induced QD Photoluminescence Enhancement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barak, Y.; Meir, I.; Shapiro, A.; Jang, Y.; Lifshitz, E. Fundamental Properties in Colloidal Quantum Dots. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Kagan, C.R.; Lifshitz, E.; Sargent, E.H.; Talapin, D.V. Building devices from colloidal quantum dots. Science 2016, 353. [Google Scholar] [CrossRef]
- Dai, X.L.; Zhang, Z.X.; Jin, Y.Z.; Niu, Y.; Cao, H.J.; Liang, X.Y.; Chen, L.W.; Wang, J.P.; Peng, X.G. Solution-processed, high-performance light-emitting diodes based on quantum dots. Nature 2014, 515, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Lesiak, A.; Drzozga, K.; Cabaj, J.; Banski, M.; Malecha, K.; Podhorodecki, A. Optical Sensors Based on II-VI Quantum Dots. Nanomaterials 2019, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Wegner, K.D.; Hildebrandt, N. Quantum dots: Bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem. Soc. Rev. 2015, 44, 4792–4834. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Cai, F.H.; Wang, D.; Wang, J.X.; Chen, J.F. Colloidal Synthesis of Semiconductor Quantum Dots toward Large Scale Production: A Review. Ind. Eng. Chem. Res. 2018, 57, 1790–1802. [Google Scholar] [CrossRef]
- Farkhani, S.M.; Valizadeh, A. Review: Three synthesis methods of CdX (X = Se, S or Te) quantum dots. IET Nanobiotechnol. 2014, 8, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Green, M. Organometallic based strategies for metal nanocrystal synthesis. Chem. Commun. 2005, 24, 3002–3011. [Google Scholar] [CrossRef]
- Gaponik, N.; Talapin, D.V.; Rogach, A.L.; Hoppe, K.; Shevchenko, E.V.; Kornowski, A.; Eychmuller, A.; Weller, H. Thiol-capping of CdTe nanocrystals: An alternative to organometallic synthetic routes. J. Phys. Chem. B 2002, 106, 7177–7185. [Google Scholar] [CrossRef]
- Silva, F.O.; Carvalho, M.S.; Mendonca, R.; Macedo, W.A.A.; Balzuweit, K.; Reiss, P.; Schiavon, M.A. Effect of surface ligands on the optical properties of aqueous soluble CdTe quantum dots. Nanoscale Res. Lett. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, D.; Gaddam, R.R.; Trinchi, A.; Cole, I. Core-shell quantum dots: Properties and applications. J. Alloys Compd. 2015, 636, 395–404. [Google Scholar] [CrossRef]
- Aldeek, F.; Balan, L.; Medjahdi, G.; Roques-Carmes, T.; Malval, J.-P.; Mustin, C.; Ghanbaja, J.; Schneider, R. Enhanced Optical Properties of Core/Shell/Shell CdTe/CdS/ZnO Quantum Dots Prepared in Aqueous Solution. J. Phys. Chem. C 2009, 113, 19458–19467. [Google Scholar] [CrossRef]
- Steckel, J.S.; Snee, P.; Coe-Sullivan, S.; Zimmer, J.P.; Halpert, J.E.; Anikeeva, P.; Kim, L.-A.; Bulovic, V.; Bawendi, M.G. Color-Saturated Green-Emitting QD-LEDs. Angew. Chem. Int. Ed. 2006, 45, 5796–5799. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Qiu, X.; Li, L.; Ren, J. Microwave-Assisted Aqueous Synthesis: A Rapid Approach to Prepare Highly Luminescent ZnSe(S) Alloyed Quantum Dots. J. Phys. Chem. B 2006, 110, 9034–9040. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Carrion, C.; Cardenas, S.; Simonet, B.M.; Valcarcel, M. Quantum dots luminescence enhancement due to illumination with UV/Vis light. Chem. Commun. 2009, 5214–5226. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Dong, Q.; Lopez, R. The Effect of Light Intensity, Temperature, and Oxygen Pressure on the Photo-Oxidation Rate of Bare PbS Quantum Dots. Nanomaterials 2018, 8, 341. [Google Scholar] [CrossRef]
- Bao, H.B.; Gong, Y.J.; Li, Z.; Gao, M.Y. Enhancement effect of illumination on the photoluminescence of water-soluble CdTe nanocrystals: Toward highly fluorescent CdTe/CdS core-shell structure. Chem. Mater. 2004, 16, 3853–3859. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Z.Y.; Correa-Duarte, M.A.; Pastoriza-Santos, I.; Giersig, M.; Kotov, N.A.; Liz-Marzan, L.M. Mechanism of strong luminescence photoactivation of citrate-stabilized water-soluble nanoparticles with CdSe cores. J. Phys. Chem. B 2004, 108, 15461–15469. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, H.G.; Riabinina, D.; Chaker, M.; Ma, D.L. Concentration-Dependent Photoinduced Photoluminescence Enhancement in Colloidal PbS Quantum Dot Solution. J. Phys. Chem. C 2010, 114, 10153–10159. [Google Scholar] [CrossRef]
- Smyntyna, V.; Semenenko, B.; Skobeeva, V.; Malushin, N. Photoactivation of luminescence in CdS nanocrystals. Beilstein J. Nanotechnol. 2014, 5, 355–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zare, H.; Marandi, M.; Fardindoost, S.; Sharma, V.K.; Yeltik, A.; Akhavan, O.; Demir, H.V.; Taghavinia, N. High-efficiency CdTe/CdS core/shell nanocrystals in water enabled by photo-induced colloidal hetero-epitaxy of CdS shelling at room temperature. Nano Res. 2015, 8, 2317–2328. [Google Scholar] [CrossRef] [Green Version]
- Freitas, L.F.; Varca, G.H.C.; Batista, J.G.D.; Lugao, A.B. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.C.; Castano, C.E.; Rojas, J.V. Supported transition metal nanomaterials: Nanocomposites synthesized by ionizing radiation. Radiat. Phys. Chem. 2017, 132, 52–64. [Google Scholar] [CrossRef]
- Dispenza, C.; Grimaldi, N.; Sabatino, M.A.; Soroka, I.L.; Jonsson, M. Radiation-Engineered Functional Nanoparticles in Aqueous Systems. J. Nanosci. Nanotechnol. 2015, 15, 3445–3467. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A. Hydrogel formation by radiation induced crosslinked copolymerization of acrylamide onto moringa gum for use in drug delivery applications. Carbohydr. Polym. 2018, 200, 262–270. [Google Scholar] [CrossRef]
- Wang, L.Q.; Magliocca, E.; Cunningham, E.L.; Mustain, W.E.; Poynton, S.D.; Escudero-Cid, R.; Nasef, M.M.; Ponce-Gonzalez, J.; Bance-Souahli, R.; Slade, R.C.T.; et al. An optimised synthesis of high performance radiation-grafted anion-exchange membranes. Green Chem. 2017, 19, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.Q.; Dai, Y.D.; Kang, B.; Han, W.; Chen, D. gamma-Radiation Synthesis of Silk Fibroin Coated CdSe Quantum Dots and Their Biocompatibility and Photostability in Living Cells. J. Nanosci. Nanotechnol. 2009, 9, 5693–5700. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Q.; Kang, B.; Dai, Y.D.; Zhang, H.X.; Chen, D. One-step fabrication of biocompatible chitosan-coated ZnS and ZnS:Mn2+ quantum dots via a gamma-radiation route. Nanoscale Res. Lett. 2011, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Q.; Chang, L.; Han, W.; Li, Z.; Dai, Y.D.; Zhang, H.Q. In situ green production of Prussian blue/natural porous framework nanocomposites for radioactive Cs+ removal. J. Radioanal. Nucl. Chem. 2018, 316, 209–219. [Google Scholar] [CrossRef]
- Chang, S.Q.; Liu, C.C.; Fu, H.L.; Li, Z.; Wu, X.; Feng, J.D.; Zhang, H.Q. Preparation of Well-Dispersed Nanosilver in MIL-101(Cr) Using Double-Solvent Radiation Method for Catalysis. Nano 2018, 13. [Google Scholar] [CrossRef]
- Withers, N.J.; Sankar, K.; Akins, B.A.; Memon, T.A.; Gu, T.Y.; Gu, J.J.; Smolyakov, G.A.; Greenberg, M.R.; Boyle, T.J.; Osinski, M. Rapid degradation of CdSe/ZnS colloidal quantum dots exposed to gamma irradiation. Appl. Phys. Lett. 2008, 93. [Google Scholar] [CrossRef]
- Stodilka, R.Z.; Carson, J.J.L.; Yu, K.; Zalman, M.B.; Li, C.S.; Wilkinson, D. Optical Degradation of CdSe/ZnS Quantum Dots upon Gamma-Ray Irradiation. J. Phys. Chem. C 2009, 113, 2580–2585. [Google Scholar] [CrossRef]
- Gaur, G.; Koktysh, D.S.; Fleetwood, D.M.; Weller, R.A.; Reed, R.A.; Rogers, B.R.; Weiss, S.M. Influence of Ionizing Radiation and the Role of Thiol Ligands on the Reversible Photodarkening of CdTe/CdS Quantum Dots. ACS Appl. Mater. Int. 2016, 8, 7869–7876. [Google Scholar] [CrossRef]
- Jovanovic, S.P.; Syrgiannis, Z.; Markovic, Z.M.; Bonasera, A.; Kepic, D.P.; Budimir, M.D.; Milivojevic, D.D.; Spasojevic, V.D.; Dramicanin, M.D.; Pavlovic, V.B.; et al. Modification of Structural and Luminescence Properties of Graphene Quantum Dots by Gamma Irradiation and Their Application in a Photodynamic Therapy. ACS Appl. Mater. Int. 2015, 7, 25865–25874. [Google Scholar] [CrossRef] [PubMed]
- Zanazzi, E.; Favaro, M.; Ficorella, A.; Pancheri, L.; Dalla Betta, G.F.; Quaranta, A. Photoluminescence enhancement of colloidal CdSe/ZnS quantum dots embedded in polyvinyl alcohol after 2 MeV proton irradiation: Crucial role of the embedding medium. Opt. Mater. 2019, 88, 271–276. [Google Scholar] [CrossRef]
- Zanazzi, E.; Favaro, M.; Ficorella, A.; Pancheri, L.; Dalla Betta, G.F.; Quaranta, A. Proton Irradiation Effects on Colloidal InGaP/ZnS Core-Shell Quantum Dots Embedded in Polydimethylsiloxane: Discriminating Core from Shell Radiation-Induced Defects through Time-Resolved Photoluminescence Analysis. J. Phys. Chem. C 2018, 122, 22170–22177. [Google Scholar] [CrossRef]
- Chang, S.Q.; Kang, B.; Dai, Y.D.; Chen, D. A novel route to synthesize CdS quantum dots on the surface of silk fibers via gamma-radiation. Mater. Lett. 2008, 62, 3447–3449. [Google Scholar] [CrossRef]
- Borchert, H.; Talapin, D.V.; Gaponik, N.; McGinley, C.; Adam, S.; Lobo, A.; Moller, T.; Weller, H. Relations between the photoluminescence efficiency of CdTe nanocrystals and their surface properties revealed by synchrotron XPS. J. Phys. Chem. B 2003, 107, 9662–9668. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.; Wu, X.; Lan, J.; Li, Z.; Zhang, X.; Zhang, H. γ-Radiation Enhanced Luminescence of Thiol-Capped Quantum Dots in Aqueous Solution. Nanomaterials 2019, 9, 506. https://doi.org/10.3390/nano9040506
Chang S, Wu X, Lan J, Li Z, Zhang X, Zhang H. γ-Radiation Enhanced Luminescence of Thiol-Capped Quantum Dots in Aqueous Solution. Nanomaterials. 2019; 9(4):506. https://doi.org/10.3390/nano9040506
Chicago/Turabian StyleChang, Shuquan, Xian Wu, Jianzhang Lan, Zheng Li, Xiaohong Zhang, and Haiqian Zhang. 2019. "γ-Radiation Enhanced Luminescence of Thiol-Capped Quantum Dots in Aqueous Solution" Nanomaterials 9, no. 4: 506. https://doi.org/10.3390/nano9040506