Fabrication and Evaluation of Lambda-Cyhalothrin Nanosuspension by One-Step Melt Emulsification Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
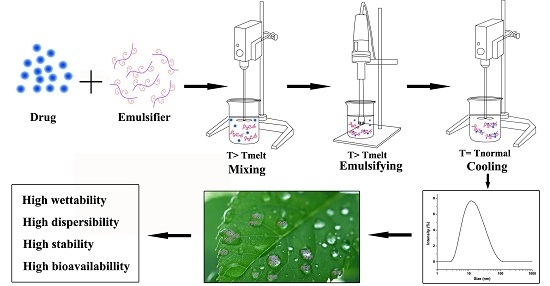
2.2.1. Preparation Procedure of LCNS
2.2.2. Particle Size Distribution (PSD)
2.2.3. Microscopic Morphology
2.2.4. Suspensibility Measurement
2.2.5. Wettability Test
2.2.6. Storage Stability
2.2.7. Bioassays
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. The Effect of Surfactants Types
3.2. The Effect of Surfactants Dosages
3.3. Microscopic Morphology Analysis
3.4. Suspensibility
3.5. Wettability and Retention
3.6. The Stability of the LCNS
3.7. Biological Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, S.Z.; Wang, M.; Chen, F.H. Research Progress and Development Prospect of Pyrethroid Pesticide. Pest 2004, 43, 289–293. [Google Scholar]
- Xu, S.C. Progress and Perspective in Research and Development of Agro-chemicals. Mod. Agrochem. 2002, 1, 7–13. [Google Scholar]
- Benner, J.P. Pesticidal compounds from higher plants. Pest. Manag. Sci. 2010, 39, 95–102. [Google Scholar] [CrossRef]
- Carvalho, F.P. Agriculture, pesticides, food security and food safety. Environ. Sci. Policy. 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Fan, A.M.; Corn, M. Pesticides and food safety. Regul. Toxicol. Pharm. 1989, 9, 158–174. [Google Scholar] [CrossRef]
- Hua, N.Z. Dvelopment and fufure of water-based pesticide formulations. Agrochemicals 2006, 45, 805–809. [Google Scholar]
- Luckham, P.F. Physical stability of suspension concentrates with particular reference to pharmaceutical and pesticide formulations. Pest. Manag. Sci. 2010, 25, 25–34. [Google Scholar] [CrossRef]
- Chin, C.P.; Wu, H.S.; Wang, S.S. New Approach to Pesticide Delivery Using Nanosuspensions: Research and Applications. Ind. Eng. Chem. Res. 2011, 50, 7637–7643. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, S.; Gokhale, R. Physical stability of nanosuspensions: Investigation of the role of stabilizers on Ostwald ripening. Int. J. Pharm. 2011, 406, 145–152. [Google Scholar] [CrossRef]
- Chan, H.K. Nanodrug particles and nanoformulations for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 405. [Google Scholar] [CrossRef]
- Kocbek, P.; Baumgartner, S.; Kristl, J. Preparation and evaluation of nanosuspensions for enhancing the dissolution of poorly soluble drugs. Int. J. Pharm. 2006, 312, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Zhang, J.J.; Gao, Y.; Zhou, J.P. Recent progress in nanosuspension. Progr. Pharm. Sci. 2007, 31, 9–14. [Google Scholar]
- Scher, H.B. Innovations and Developments in Pesticide Formulations: An overview. In Pesticide Formulations: Innovations and Developments; Scher, H.B., Cross, B., Eds.; American Chemical Society: Washington, DC, USA, 1988; Volume 371, pp. 1–5. [Google Scholar]
- Ely, D.R.; García, R.E.; Thommes, M. Ostwald-Freundlich diffusion-limited dissolution kinetics of nanoparticles. Powder Technol. 2014, 257, 120–123. [Google Scholar] [CrossRef]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Hong, W.; Cerna, C. Effect of nanonization on absorption of 301029: Ex vivo and in vivo pharmacokinetic correlations determined by LC/MS. Pharm. Res. 2002, 19, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, P.; Kumar, G.A. Nanosuspension technology: A review. Int. J. Pharm. Pharm. Sc. 2010, 2, 35–40. [Google Scholar]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. 2011, 2, 81–87. [Google Scholar]
- Kakran, M.; Sahoo, N.G.; Li, L. Fabrication of drug nanoparticles by evaporative precipitation of nanosuspension. Int. J. Pharm. 2009, 383, 285–292. [Google Scholar] [CrossRef]
- Pan, Z.; Cui, B.; Cui, H.X.; Pan, H.Y. Progress on pesticide nanosuspension and its preparation methods. Chin. J. Pestic. Sci. 2014, 16, 635–643. [Google Scholar]
- Bhakay, A.; Vizzotti, E.; Li, M. Incorporation of Fenofibrate Nanoparticles Prepared by Melt Emulsification into Polymeric Films. J. Pharm. Innov. 2016, 11, 53–63. [Google Scholar] [CrossRef]
- Pan, Z.; Cui, B.; Zeng, Z.Z.; Feng, L.; Liu, G.Q.; Cui, H.X.; Pan, H.Y. Lambda-Cyhalothrin nanosuspension prepared by the melt emulsification-high pressure homogenization method. J. Nanometer 2015, 16, 263. [Google Scholar] [CrossRef]
- Knieke, C.; Rawtani, A.; Davé, R.N. Concentrated Fenofibrate Nanoparticle Suspensions from Melt Emulsification for Enhanced Drug Dissolution. Chem. Eng. Technol. 2014, 37, 157–167. [Google Scholar] [CrossRef]
- Zhang, B.H.; Yin, P.J.; Wang, C.; Zhang, W.J.; Gao, M.M.; Xie, D.D.; Yu, W.Y. Effects of Formulation Adjuvants for Difenoconazole SC on Wetting Behavior and Retention on Plant Leaves. Agrochemicals 2015, 54, 736–739. [Google Scholar]
- Yuan, H. Study on the Point of Run-off and the Maximum Retention of Spray Liquid on Crop Leaves. Chin. J. Pestic. Sci. 2000, 2, 66–71. [Google Scholar]
- Young, R.D.F.; Thacker, J.R.M.; Curtis, D.J. The effects of three adjuvants on the retention of insecticide formulations by cabbage leaves. J. Environ. Sci. Heal. B 1996, 31, 165–1778. [Google Scholar] [CrossRef]
- Ahuja, B.K.; Jena, S.K.; Paidi, S.K. Formulation, optimization and in vitro-in vivo evaluation of febuxostat nanosuspension. Int. J. Pharm. 2015, 478, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Zhang, L.; Ren, T.R. Surface and Interfacial Properties of Poly Alkyl Phenol Polyoxyethylene Ether-type Surfactants. World Pesticides 2014, 36, 50–54. [Google Scholar]
- Bergström, L. Hamaker constants of inorganic materials. Adv. Colloid Interface 1997, 70, 125–169. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef]
- Cerdeira, A.M.; Mazzotti, M.; Gander, B. Miconazole nanosuspensions: Influence of formulation variables on particle size reduction and physical stability. Int. J. Pharm. 2010, 396, 210–218. [Google Scholar] [CrossRef]
- Rao, S.; Song, Y.; Frank, P. Particle size reduction to the nanometer range: A promising approach to improve buccal absorption of poorly water-soluble drugs. Int. J. Nanomed. 2011, 6, 1245–1251. [Google Scholar]
- Donoso, M.D.; Haskell, R.J.; Schartman, R.R. Surfactant choice and the physical stability of nanosuspensions as a function of pH. Int. J. Pharm. 2012, 439, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.H. Interacting behavior between amino sulfonate amphoteric surfactant and octylphenol polyoxyethylene ether (7) in aqueous solution and pH effect. J. Ind. Eng. Chem. 2014, 20, 3649–3657. [Google Scholar] [CrossRef]
- González, F.G.; Vilchez, M.A.C.; Hidalgo-Alvarez, R. Adsorption of anionic surfactants on positively charged polystyrene particles II. Colloid Polym. Sci. 1991, 269, 406–411. [Google Scholar] [CrossRef]
- Liu, L.L.; Wang, Y. The Influence of Surfactant on the Size Control and Shape of Nano Materials. J. Changchun Normal Univ. 2014, 33, 62–64. [Google Scholar]
- Grau, M.J.; Kayser, O.; Müller, R.H. Nanosuspensions of poorly soluble drugs- reproducibility of small scale production. Int. J. Pharmaceut. 2000, 196, 155–159. [Google Scholar] [CrossRef]
- Chingunpitak, J.; Puttipipatkhachorn, S.; Chavalitshewinkoon-Petmitr, P.; Tozuka, Y.; Moribe, K.; Yamamoto, K. Formation, physical stability and in vitro antimalarial activity of dihydroartemisinin nanosuspensions obtained by co-grinding method. Drug Dev. Ind. Pharm. 2008, 34, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, L.; Skantze, P.; Skantze, U.; Westergren, J.; Olsson, U. Amorphous Drug Nano- suspensions. 3. Particle Dissolution and Crystal Growth. Langmuir 2007, 23, 9866–9874. [Google Scholar] [CrossRef]
- Bajpai, M.; Agarwal, V. Stability Issues Related to Nanosuspensions: A Review. Pharm. Nanotechnol. 2013, 1, 85–92. [Google Scholar]
- Liu, Z.H.; Wei, H.H.; Li, Y.G.; Li, S.L.; Zhang, L.; Chen, H.L. Effects of milling and surfactants on suspensibility and spore viability in Paenibacillus polymyxa powder formulations. Biocontrol Sci. Technol. 2011, 21, 1103–1116. [Google Scholar] [CrossRef]
- Chung, H.S.; Hogg, R. The effect of brownian motion on particle size analysis by sedimentation. Powder Technol. 1985, 41, 211–216. [Google Scholar] [CrossRef]
- Sharma, N.N.; Mittal, R.K. Brownian motion model of nanoparticle considering nonrigidity of matter-a systems modeling approach. IEEE Trans. Nanotechnol. 2005, 4, 180–186. [Google Scholar] [CrossRef]
- Kadota, G.; Matsunaka, S. Effect of Surfactants on Foliar Wettability in Rice Plants. J. Pest. Sci. 1986, 11, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Baldridge, J.W.; Podella, C.W. Reduction of Surface Tension, Interfacial Tension, and Critical Micelle Concentration Using a Protein-Based Surfactant Synergist. US WO/2007/100411, 7 September 2007. [Google Scholar]
- Muherei, M.A.; Junin, R. Investigating Synergism in Critical Micelle Concentration of Anionic-Nonionic Surfactant Mixtures: Surface versus Interfacial Tension Techniques. Asian J. Appl. Sci. 2009, 2, 115–127. [Google Scholar] [CrossRef]
- Yamada, T.; Saito, N.; Imai, T.; Otagiri, M. Effect of grinding with hydroxypropyl cellulose on the dissolution and particle size of a poorly water-soluble drug. Chem. Pharm. Bull. 1999, 47, 1311–1313. [Google Scholar] [CrossRef] [PubMed]
- Mohanachandran, P.S.; Sindhumol, P.G.; Kiran, T.S. Review: Enhancement of solubility and dissolution rate: An overview. Pharm. Glob. Int. J. 2010, 4, 1–10. [Google Scholar]
- Müller, R.H.; Peters, K. Nanosuspensions for the formulation of poorly soluble drugs: I. Preparation by a size-reduction technique. Int. J. Pharmaceut. 1998, 160, 229–237. [Google Scholar] [CrossRef]
- Du, H.; Xu, S.H.; Sun, Z.W. Effect of the Hydrodynamic Radius of Colloid Microspheres on the Estimation of the Coagulation Rate Constant. Acta Phys.-Chim. Sin. 2010, 26, 2807–2812. [Google Scholar]
- Sanson, N.; Bouyer, F.; Destarac, M.; In, M.; Gerardin, C. Hybrid polyion complex micelles formed from double hydrophilic block copolymers and multivalent metal ions: Size control and nanostructure. Langmuir 2012, 28, 3773–3782. [Google Scholar] [CrossRef]
- Rabinow, B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef]
- Tadros, T.F. Control and assessment of the physical stability of pesticidal suspension concentrates. Chem. Ind. 1980, 57, 211–218. [Google Scholar]
- Niessen, H.J. Importance of storage stability studies in the development of pesticide formulations. Pest. Manag. Sci. 2010, 6, 181–188. [Google Scholar] [CrossRef]
- Verma, S.; Lan, Y.; Gokhale, R. Quality by design approach to understand the process of nanosuspension preparation. Int. J. Pharmaceut. 2009, 377, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Chin, B.D.; Yang, S.M. Surfactant Effect on the Stability and Electrorheological Properties of Polyaniline Particle Suspension. J. Colloid Interface Sci. 1998, 206, 424–438. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, M.; Nakamura, T.; Muraoka, E. Preparation of Binapacryl Suspension Concentrates and Their Physical Stability. J. Pestic. Sci. 1982, 7, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, R.L.; San Martin, M.A.L.; Ganen, R.A.; Quintanar, G.D. Experimental model for the teaching of the interfacial properties and physical stability of pharmaceutical suspensions. Rev. Mex. Cienc. 2003, 34, 8–20. [Google Scholar]
- Shete, G.; Jain, H.; Punj, D.; Prajapat, H.; Akotiya, P.; Bansal, A.K. Stabilizers used in nanocrystal based drug delivery system. J. Excip. Food Chem. 2014, 5, 184–209. [Google Scholar]
- Cui, B.; Feng, L.; Pan, Z.Z.; Yu, M.L.; Zeng, Z.H.; Sun, C.J.; Zhao, X.; Wang, Y.; Cui, H.X. Evaluation of Stability and Biological Activity of Solid Nanodispersion of Lambda-Cyhalothrin. PLoS ONE 2015, 10, e0135953. [Google Scholar] [CrossRef]
- Streibig, J.C. Herbicide bioassay. Weed. Res. 1988, 28, 479–484. [Google Scholar] [CrossRef]
- Anjali, C.H.; Sudheer, K.S.; Margulis-Goshen, K.; Magdassi, S.; Mukherjee, A.; Chandrasekaran, N. Formulation of water-dispersible nanopermethrin for larvicidal applications. Ecotox. Environ. Saf. 2010, 73, 1932–1936. [Google Scholar] [CrossRef]
- Anjali, C.H.; Sharma, Y.; Mukherjee, A.; Chandrasekaran, N. Neem oil (Azadirachta indica) nanoemulsion-a potent larvicidal agent against Culex quinquefasciatus. Pest. Manag. Sci. 2012, 68, 158–163. [Google Scholar] [CrossRef] [PubMed]










| Surfactant | Mean Size (nm) | D90 (nm) | PDI |
|---|---|---|---|
| MRES | 46.1 ± 0.5 de | 209.3 ± 8.1 b | 0.421 ± 0.003 b |
| OP-10 | 42.2 ± 0.4 e | 223.3 ± 74.8 b | 0.491 ± 0.037 b |
| NP-7 | 37.1 ± 0.2 e | 188.0 ± 38.0 b | 0.418 ± 0.029 b |
| Emulsifier 700 | 12.0 ± 0.1 e | 39.9 ± 0.5 c | 0.279 ± 0.135 b |
| Span 80 | 105.1 ± 4.1 cd | 323.0 ± 154.8 b | 0.332 ± 0.223 b |
| Emulsifier 600 | 140.4 ± 9.8 c | 187.0 ± 41.5 b | 0.441 ± 0.329 b |
| Emulsifier 1601 | 220.6 ± 13.8 b | 247.3 ± 30.5 b | 0.913 ± 0.150 a |
| Tween 80 | 565.5 ± 95.7 a | 496.0 ± 119.4 a | 1.000 ± 0.000 a |
| Ratio of Pesticide to Emulsifier 700 | Mean Size (nm) | D90 (nm) | PDI |
|---|---|---|---|
| 20/1 | 48.6 ± 0.8 a | 207.6 ± 13.7 a | 0.412 ± 0.016 a |
| 20/2 | 22.2 ± 0.3 b | 84.8 ± 3.4 b | 0.357 ± 0.003 b |
| 20/3 | 12.0 ± 0.1 e | 39.9 ± 0.5 d | 0.279 ± 0.019 d |
| 20/4 | 16.8 ± 0.1 c | 59.6 ± 5.4 c | 0.313 ± 0.007 c |
| 20/5 | 15.2 ± 0.1 d | 50.8 ± 2.4 cd | 0.279 ± 0.016 d |
| Formulation | Toxicity Regression Equation | Correlation Coefficient | LC50 (mg/mL) | 95% Confidence Limit |
|---|---|---|---|---|
| LCNS | y = 0.8981x + 5.7229 | 0.9235 | 0.1566 | 0.0657–0.2697 |
| SC-A | y = 1.1891x + 5.6716 | 0.9429 | 0.4177 | 0.3007–0.6487 |
| SC-B | y = 1.0222x + 5.5773 | 0.9598 | 0.2724 | 0.1457–0.4145 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Cui, B.; Guo, L.; Wang, A.; Zhao, X.; Wang, Y.; Sun, C.; Zeng, Z.; Zhi, H.; Chen, H.; et al. Fabrication and Evaluation of Lambda-Cyhalothrin Nanosuspension by One-Step Melt Emulsification Technique. Nanomaterials 2019, 9, 145. https://doi.org/10.3390/nano9020145
Wang C, Cui B, Guo L, Wang A, Zhao X, Wang Y, Sun C, Zeng Z, Zhi H, Chen H, et al. Fabrication and Evaluation of Lambda-Cyhalothrin Nanosuspension by One-Step Melt Emulsification Technique. Nanomaterials. 2019; 9(2):145. https://doi.org/10.3390/nano9020145
Chicago/Turabian StyleWang, Chunxin, Bo Cui, Liang Guo, Anqi Wang, Xiang Zhao, Yan Wang, Changjiao Sun, Zhanghua Zeng, Heng Zhi, Hongyan Chen, and et al. 2019. "Fabrication and Evaluation of Lambda-Cyhalothrin Nanosuspension by One-Step Melt Emulsification Technique" Nanomaterials 9, no. 2: 145. https://doi.org/10.3390/nano9020145